Estimation of Rutin and Quercetin in Amaranthus viridis L by High Performance Layer Chromatography (HPLC)
December 16, 2024
Ashok Kumar, B.S1*., Lakshman, K2., Jayaveera, K.N3., Nandeesh, R4., Mani Tripathi, S.N1., Vamshi Krishna, N1., Manjunath, M5 and Suresh, M.V5.
1Department of Pharmacognosy, Sri K.V.College of Pharmacy, Chickballapur, Karnataka (INDIA). 2Department of Pharmacognosy, PES College of Pharmacy, Bangaluru, Karnataka (INDIA). 3Department of Chemistry, Jawaharlal Nehru Technological University, Anantapur, Andhra Pradesh (INDIA). 4Department of Pharmacognosy, Sree Siddhaganga college of Pharmacy, Tumkur, Karnataka 5Department of Pharmacognosy, Rural College of Pharmacy, Devanahalli, Karnataka (INDIA)
Issued 01 April 2009
Abstract
Flavonoids are a group of polyphenolic compounds, which are widely distributed through out the plant kingdom. Flavonoids like Rutin and quercetin possess many biochemical effects like inhibition of enzymes, regulatory role on different hormones and pharmacological activities like antimicrobial, antioxidant, and anticancer, antihepatotoxic, protection of cardio vascular system. An HPLC method was developed for the estimation of rutin and quercetin from methanol herbal extract of Amaranthus viridis. Amaranthus viridis is traditionally used for treatment of constipation, inflammation, eczema, bronchitis, anemia, and leprosy.
Keywords: Amaranthus viridis, Rutin, quercetin, antioxidant activity, HPLC.
Introduction
Amaranthus viridis Linn, belongs to family Amaranthaceae and is traditionally used for treatment of constipation, inflammation, eczema, bronchitis, anaemia, and leprosy (Kirtikar, and Basu, 1987, Sivarajan and Balachandran. 1994, Anonymous, 1996). Flavonoids are a group of polyphenolic compounds, which are widely distributed through out the plant kingdom. To date about 300 varieties of flavonoids are known (Anonymous, 1996). Many have low toxicity in mammals and some of them are widely used in medicine for maintenance of capillary integrity (Kuhnau, 1976). Rutin, 5,7,3,4, tetrahydroxy flavonol -3-rhamnoglucoside and quercetin 5,7,3,4,- tetrahydroxy flavonol are exhibits anti-inflammatory, antihepatotoxic (Cesarone,1992), antiulcer (Clack et al., 1950), antiallergic, antiviral actions and some of them provides protection against cardiovascular mortality (Colergie Smith et al., 1980, Hertog, et al., 1993). Both rutin and quercetin possess antioxidant activity and reduce low density lipoproteins (LDL) oxidation (De-Whalley, et al., 1990), quercetin in combination with other flavonoids, are inhibiting a number of enzymes like bradykinin (Bamard et al., (1993), tyrosine kinase (Hur, et al., (1994), and 5-nucleotidase activity (Beladi, et al., 1987). Rutin and quercetin have shown regulatory activity of hormones like affect the transport, metabolism and action of thyroid hormones. High Performance Layer Chromatography (HPLC) method is the suitable method for estimation of chemical constituents present in plant materials. Hence Amaranthus viridis contains rutin and quercetin are important active constituents and is estimated by HPLC method.
Methodology
Instrumentation
The Shimadzu class LC-10AT HPLC, Hichrom C18 and a Rheodyne 7725i injector fitted with a 20 ml loop, column oven, and a photodiode array detector. The output signal was monitored and processed using chromquest version3.0 software on Pentium computer (Hewlett Packard).
Solvent and Chemicals
Rutin and quercetin obtained from natural remedies (Bangalore) chromatographic grade methanol, formic acid and acetonitrile (AR), were obtained from Merck (Mumbai, India).
Extraction of Plant material
Plant material was collected from Chickballapur surroundings and was authenticated by Dr. B.K.Vekatesh, Department of Botany, Government First Grade College, Chickballapur. Specimen was stored in college herbarium (SKVCP-20). Amaranthus viridis leaves were extracted with distilled Methanol by Soxhlet apparatus. The pooled Methanolic extract was evaporated under vacuum to dryness, yielding was noted. Methanolic extract was subjected for estimation of rutin and quercetin.
Preparation of Standard and Sample Solutions
Rutin and quercetin 10 mg were accurately weighed into a 10 mL volumetric flask, dissolved in 5 mL methanol and the solution was made up to 10 mL with the same solvent (1 mg/mL). A. viridis fruit extract was accurately weighed (10 mg) into a 10 mL of volumetric flask and shaken on a mechanical shaker for 10 min filtered through Whatman filter paper No. 42 and the filtrate was used for analysis.
Chromatography
Flow rate : 0.9 ml/min
Detection : 340 nm
Injection quantity : 50 ml
Column used : Hichrom C18 (150 mmx4.6 mm i.d., 5m)
Column temperature : 35C
Mobile phase ration :70:30 % v/v
Mobile phase : 0.5% Formic acid: Acetonitrile
Results and Discussion
The retention time of standards rutin and quercetin were found to be 4.072 and 19.104 (Graphs1). The retention time of rutin and quercetin in Amaranthus viridis were found to be 4.715 and 19.051 (Graphs2), which are matching with standard Rt values respectively. Then the amount of rutin and quercetin in Amaranthus viridis was found to be 58.52 and 9.12 % w/v respectively graphs
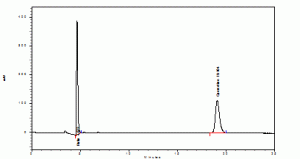
Graph 1: Standards (Rutin and Quercetine)
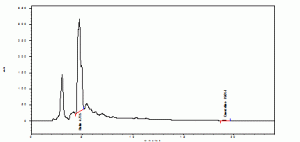
Graph 2: Methanolic extract of Amarnthus Viridis Linn.
Conclusion
Amaranthus viridis is widely used as spinach which is traditionally used to treate constipation, inflammation, eczema, bronchitis, anaemia, leprosy. This study shows the presence of rutin and quercetine in A. viridis. Plant may posses to have good medicinal food.
Acknowledgment
The authors are thankful to Sri K.V.Naveen Kiran, Chairman, Sri K.V.College of Pharmacy, Chickballapur, Karnataka (INDIA) for providing required facilities.
References
Anonymous. 1996. Wealth of India, publications and information directorate, CSIR, New Delhi, Vol. I, 1988, 217 Vol II. Ministry of Health & Family Welfare. Govt. of India, Controller of Publications, New Delhi, 53 – 54.
Bamard D.L., Smee D.F., Huffman J.H., Meyerson C.R. and Sidwell R.W., 1993. Review of Quercetin and related Bioflavonoids. Chemotherapy, 39; 203-11.
Beladi I., Musci R., Pusztai M., Bakay I., 1987. Rosztoczy M. and Gabor, Bioactivity of flavonoids. Stud Org Chem, 57; 42-50.
Cesarone M.R., Laurora G., Ricci A., Belcaco G. and Pomante P., 1992. Acute effects of hydroxyethylrutosides on capillary filtration in normal volunteers, patients with various hypotensions and in patients with diabetic micro angiopathy. J Vas Disease 21; 76-80.
Clack W., Heller W., Michel C. and Saran M. 1950. Effect of flavonoid substances on histamine toxicity, anaphylactic shock and histamine-enhanced capacity to dye, Journal of Allergy, 21: 133-147.
Colergie Smith P,O., Thomas P., Scurry J.H. and Dormandy JA. 1980. Causes of various ulceration, a New Hypothesis. Br Med J, 296; 1726-7.
De-Whalley C., Rankin S, M., Houct J.R.S., Jessup W. and Leake D.S. 1990. Flavonoids inhibit the oxidative modification of low-density lipoproteins by macrophages. Biochem Pharmacol. 39; 1743-50.
Hertog M.G.L., Hollman P.C.H., Katan Klohout D., 1993. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in the Netherlands. Nutr Cancer. 20; 21-29.
Hur C.Q., Chen K., Shi Q., Kikushkie R.E., Cheng Y.C. and Lee K.H., 1994. Apoptosis of HIV infected cell following treatment with Shosoikorso, J Nat Pro, 57; 42-50.
Harbone J.B., 1984. Phytochemical Methods, Chapman and Hall, 2nd edition, New York, 1-31.
Kirtikar K.R. and Basu B.D. 1987. Indian Materia Medica, International book distributors, Dehra Dun, India, 3; 2055-57.
Kuhnau J., 1976. The flavonoids: A class of semi-essential food components: their role in human nutrition. World Res. Nut Diet, 24; 17-91.
Sivarajan V.V. and Balachandran I., 1994. Ayurvedic Drugs and Their Plant Sources. Oxford and India Book House Publishing Co., Pvt. Ltd., New Delhi, 473-474.