|
Ethnobotanical Leaflets 13: 1168-85. 2009.
Seed Germination, Foliar Vestiture and Stem Anatomical Features of Urena lobata L. (Malvaceae): An Ethnobotanical and/or Ethnomedicinal Plant Genetic Resource in Nigeria
Gordian C. Obute and Tessy O. Nwaru
Department of Plant Science and Biotechnology, University of Port Harcourt, Choba Port Harcourt, Nigeria E-mail:
Issued 01 September 2009
Abstract
The seed germination enhancement, foliar vestiture studies and stem anatomical features of Urena lobata L. were investigated against the backdrop of its ethnobotanical and ethnomedicinal applications. Results show that the plant branches profusely thus yielding much of the vegetative parts used for various purposes. Germination of the seeds was enhanced by mechanical scarification and sulphuric acid treatments and the acid treatment elicited greater effects than the mechanical scarification. Leaf morphology especially the type and depth of lobes varied within the species while stellate and sharply pointed trichomes were observed on the abaxial and adaxial foliar surfaces. The possible roles of the glandular trichomes as secretory receptors of the active ingredients that impart medicinal efficacy to the plant were highlighted. Anatomical investigations of the stem showed that while the petiole is glabrous, the stem was covered with sharply pointed trichomes. Key words: Glandular, trichome, ethnomedicine, germination.
Introduction
Urena L. is a small genus of about seven species widely distributed throughout the tropics and subtropics of both the northern and southern hemispheres. A member of this genus is Caesar weed, Urena lobata L., Family Malvaceae and Order-malvales (Gill, 1988). It occurs in Nigeria and has been associated with several synonyms including: U. americana L.F., U. grandiflora DC, U. trilobata Vell, U. diversifolia Schmmach (ISB, 2003). The FAO (1996) country report lists the plant as one of the under-researched plant genetic resources in Nigeria. It grows abundantly around the western and eastern states (Fig.1), with little distribution in the drier savannah regions (Purseglove, 1968). U. lobata grows well at elevations up to 1500m above sea level (PIE, 2003). Its national ethnobotanical importance is expressed in the names given it by several tribal enclaves (Irvine, 1963) because of its varied uses. Some of the common tribal names for the species are: Tribe Common Name Igbo Udo agheregha or Udo azuzo Yoruba Akeri, ake-riri or Ii-omode Hausa Ramaniya, rana-ram or kafi-rama Benin Oronhon Efik Ndin
Hutchinson and Dalziel (1958) described the plant as a fibrous undershrub with stellate hairs; leaves very variable, usually more or less 3-5-lobed, sometimes deeply so, whitish beneath; middle nerve often with a large pitted gland near the base, flower axillary, usually solitary, epicalyx of 5 linear-lanceolate bracteoles united at the base and persistent in fruit; fruit depressed-globose, covered with rigid hooked burs; flowers rose-pink or yellow. PlantNet (2009) recorded that U. lobata leaves are ovate, lanceolate or ± circular in shape, 510 cm long, 35 cm wide, deeply lobed, margins toothed, and the abaxial surface with a prominent gland on the midrib near the junction with the petiole; petioles are up to 8 cm long. Epicalyx 57 mm long, fused for about half their length. Calyx shorter than or as long as the epicalyx. Petals 1020 mm long, pink. Fruits about 10 mm in diameter, carpels about 6 mm long, spinescent. Some researchers recognize the form with deeply lobed leaves in which the sinuses are rounded rather than acute (Wagner et al., 1999) as a separate species or a variety called U. sinuata. This view, however, is not widely held by other researchers. (Stone, 1970) described the plant as an erect subshrub, of up to 1 m tall; leaves palmately lobed or angled, downy grayish pubescent with stellate hairs, 4-8 cm long, usually 5-lobed with the lobes again pinnatifid, or sometimes subentire but angled; flowers clustered; corolla pinkish-violet, about 1 cm broad; fruit pubescent and beset with hooked bristles (glochidia). Extracts of its leaves and roots are used in folk medicine to treat diverse ailments including colic, malaria, gonorrhea, fever, open wounds, toothache and rheumatism (FRIM, 2003). A semi purified glycoside obtained from the leaves is reported to be 86% as effective an anti-inflammatory as aspirin in rats (Bautista, 2000). The raw leaves contain 81.8% moisture, 54 cal, 3.2 of protein, 0.1g fat, 12.8g carbohydrates, 1.8g fiber and 2.1g ash, 55mg calcium and 67mg of phosphorus per 100g (FAO, 2003). Fiber produced from this plant is used in making coffee and jute bags (Howes, 1979). The plant, however, has a nuisance factor because the fruits are armed with burrs that make them stick on to human clothing and animal fur. Its colourfully attractive flowers add to the aesthetics of areas it has colonized. U. lobata is listed as an invasive species or obnoxious weed (FLEPPC, 1999) irrespective of its numerous ethnobotanical and ethnomedicinal uses. Literature is scanty on its cytoanatomical features, particularly those of the leaf, although these may contribute immensely to its specific characterization and diagnosis. Similarly, the cytotaxonomic aspects of the plant are yet to be documented against the backdrop of rapid genetic erosion of germplasm resources, and the need to document and conserve ecologically and ethnobotanically useful but neglected species. We undertook to study the seed germination patterns, morphological and anatomical characters of the leaf and stem with a view to adding to the list of standard descriptors for the plant. Materials and Methods Seeds of U. lobata were collected from the Botanical Garden of University of Port Harcourt into marked polyethylene bags and were later sorted to remove obviously damaged ones and stored in a freezer until required for the study. Germination studies For the germination trials, seed lots of 50 each were subjected to viability tests before using them for the study. Different lots of 35 viable seeds were mechanically scarified by puncturing the seed coats with razor blades before sowing them in moistened folds of filter paper in Petri dishes. Another batch was subjected to chemical treatment by pre-soaking in concentrated sulphuric acid (Ogunwenmo and Ugborogho, 1999) for 10 minutes before assaying germination as with the protocol above. Seeds soaked with ordinary water between fold of filter paper in Petri dishes served as controls. To ascertain the effects of different soaking periods in sulphuric acid, some seeds were pre-soaked for various time regimes, washed with distilled water and assayed for germination as in the protocols outlined above. Macro morphological features
The control seedlings were allowed to grow to maturity and their gross morphological features were monitored. The number of branches per plant was scored; and angles of branching for primary, secondary and tertiary branches were measured with a pair of compass and protractor. Other features like leaf shape and type were visually scored for 10 plants. Micro morphological Features Leaf Epidermis Rectangular cuttings were taken from the median portions (Olowokudejo, 1990) of mature leaves with razor blades and the surfaces were cleared by flooding with 5% Sodium hypochlorite (domestic bleach) for about 1hr. The treatment bleached the chlorophyll content to allow for separation of the epidermal surfaces. Epidermal stripes were obtained by mechanical scaring with a razor blade; while residues of loose tissues were brushed off with the aid of a soft camel hair brush and washed with distilled water (Cutler, 1978). The procedure was carried out for both leaf surfaces. Then the clear epidermal stripes were washed in several changes of distilled water, stained in 1% safranin for 2 minutes and temporarily mounted in glycerin. The preparations were examined with a Leitz Labolux light microscope at x10 and magnification to note the trichome types and distribution. Anatomical studies of the stem and petiole Leaf petiole and stem cuttings were fixed in formalin acetic acid alcohol mixture [(F.A.A. - 1 part formaldehyde, 1 part glacial acetic acid and 18 parts 70% ethanol (v/v)]. The samples were dehydrated through a graded series of alcohol (30%, 50%, 70%, and 95% with 2 changes). The preparations were stained in safranin green (Johnsen, 1940) and several free-hand sections of the stem and petiole were made and the thinnest sections were mounted on clean slides and examined under a Leitz Labolux microscope. Photomicrographs of views reflecting the best anatomical sections were taken. Results and Discussion
Germination study.
Mechanical scarification enhanced germination percent of up to 70% after 5 days of planting as against the less than 10 % observed for the unscarified seed lot (Fig 2a). Similarly, seed treatment with sulphuric acid enhanced germination % up to 65% on the second day compared with non-treated seeds at less than 5% (Fig.2b). Apparently the mechanical scarification treatment boosted seed germination more than the acid treatment. Pre-soaking in dilute sulphuric acid for 30minutes gave the best germination of up to 95% as opposed to pre-soaking for shorter or longer periods (Fig. 2c). Macromorphology
Number and angles of branches The stems were profusely branched from the secondary and tertiary branches after the first (primary) branching. Number of primary branches per plant ranged from 16-14. The secondary branches ranged from 4 45 and tertiary branches ranged from 2-13 per plant. The angle of branching ranged from 550 300 in the secondary ranged from 300 560 and the tertiary from 4O12O. The variation in the angle and number of branches per plant is shown in Table 2. Leaf type/ Petiole length
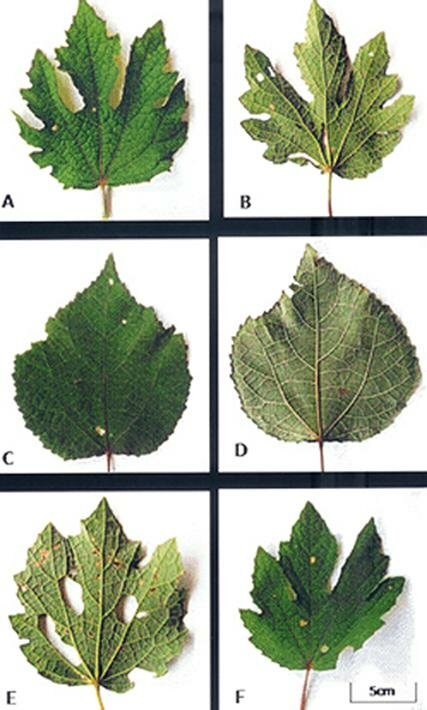
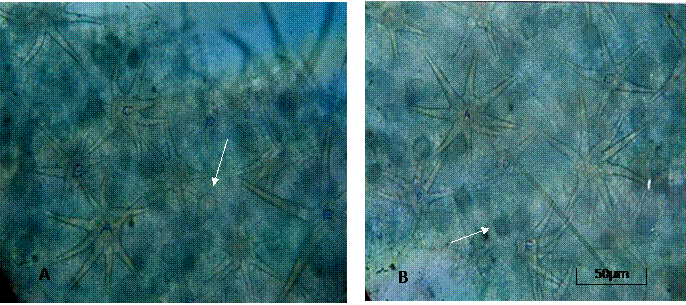
The leaves were simple, ovate, and angled or shallowly lobbed with serrate margins and variants of these shapes were observed. Plate 1 shows these variations in leaf shape and margin features including deeply lobed (Plate1A), deeply palmatedly lobed (Plate 1B), ovate with serrate margin (Plate 1C) circular with shallowly depressed margin (Plate 1D), ovate shallowly lobed (Plate 1E) and palmate shallowly lobed (Plate 1F). Length and breadth dimensions of the simple and angled or shallowly lobbed leaves (Table 2) varied according to the stage of development of the leaf. Logically the young leaves of both the simple and angled ones showed a narrow range compared with the mature types. The petiole length for the simple young leaf ranges from 0.3 - 0.7cm while the angled lobbed for young ranges from 0.3 - 0.7cm. Also the simple mature range from 0.6 3.4 cm while the angled, lobbed range from 3.1- 5.3cm. Foliar Vestiture Simple globular glandular and stellate trichomes were observed side by side on both the adaxial and abaxial leaf epidermal surfaces (Plates 1A, B) and these occurred more abundantly on the abaxial than the adaxial leaf epidermis. The stellate type was of various forms ranging from 2 9 sharply-armed trichomes. The arms were uniseriate with slightly bulging basal cells co-joined at the points of attachment to the leaf surface (Plate 2). Some were larger in size than others and tended to be loosely layered thus overlapping the smaller stellate and globular trichomes. The glandular trichomes (arrowed) were obviously stalked with spherical heads and some (especially on the adaxial surface) appeared slightly reniform in shape (Plate 2B). Transverse section through the petiole and stem.
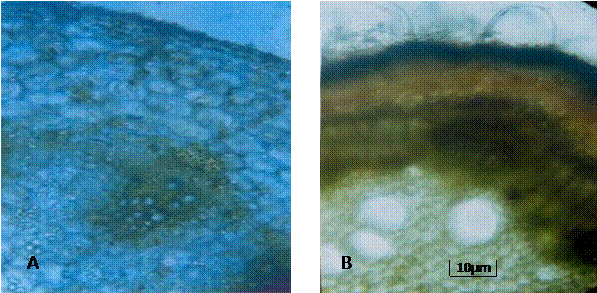
The transverse section of the petiole and stem of U. lobata show that the epidermal layer in both was biserriate (Plate 3A). The cortex directly underlies the epidermis and comprises elongated angular parenchyma cells. The vascular bundles appear in the regular ring form of dicotyledonous plants. While there were no hairs on petiole epidermis that of the stem was covered with uniseriate hairs (Plate 3B). Germination Seeds of U. lobata appear to exhibit a dormancy complex. Results showed that without enhancement, the germination % within a seven-day period is about 5% (Figs. 2 a and b) as against 70% and 65% (Figs. 2 a and b) recorded for mechanical and sulphuric acid treatments respectively. We observed as well that pre-soaking the seeds in dilute sulphuric acid for 30 minutes promoted germination% up to 95% (Fig.1c). It follows that removal of dormancy factors with chemical (Harris, 1986) and mechanical treatments largely promotes germination in the species. This is significant because efforts at domestication and establishment of farms for sustainable use of the crop for medicinal and or industrial purposes. Macromorphology. The angles of branching and number branches in this plant are identifying features that separate it from related species. It is a reflection of the bushiness of the plant since the branching turned profuse from the secondary to the tertiary levels. The average number of branches was highest at the secondary level (Table 1) and is most likely a genetic expression of the phylotaxy of the species. Obute and Omotayo (1999) used these features to separate forms of Hibiscus rosa-sinensis. In describing the species these features are useful descriptors since they determine the unique physiographic features that aid its quick identification alongside the reproductive taxonomic characters. There appears to be a connection between the angle of branching, number of branches and the orientation of leaves for maximal reception of incident insolation for metabolic activities. Whereas the secondary branches were more numerous probably to achieve greater spread, the angle of branching (Table1) was also wider at the secondary level than the others. The tertiary angle on the contrary narrowed to maintain the erect structure of the plant. Wide variation was observed in the shape of the leaves (Plate 1). This is in line with the description of leaves as very variable (Hutchison and Dalziel, 1958). For a medicinal plant, this is an important diagnostic feature. Descriptions of the leaves as 5-lobed were not observed in the material studied. However, it buttresses the wide variation in the lamina types for the species. A variable feature like this may be environmentally rather than genetically controlled and thus might not serve confirmatory diagnostic roles. Petiole length was also variable and appears to follow after the nature of the shapes. For the young leaves the range in petiole length was narrow (Table 3) compared with the older petioles. Trichomes We observed 5 9 armed stellate as well as glandular trichomes on both upper and lower leaf surfaces of Urena lobata (Plate 3). This is within the range of reported number of arms or branches for members of Malvaceae that produce stellate trichomes. The adaxial surfaces were covered with more of this than the abaxial. Presence of glandular trichomes suggests storage of secretions or secretion of secondary metabolites useful in the life adaptations of a plant. Glandular trichomes have been associated with presence of chemicals that may impart medicinal properties to plants (Celka et al., 2006). This may be the case here, although histochemical analysis of these glandular structures is necessary to ascertain their functions on the leaves of U. lobata. Vestiture in flowering plants is useful in establishing systematic relations (Metcalfe and Chalk, 1950) and may serve as important diagnostic features of ethnomedicinal plants. It has severally been used to delineate closely related taxa including Telfairia occidentales (Okoli, 1987), Boerhavia spp. and Aneilema spp. (Edeoga and Ikem, 2001; Edeoga and Ogbebor, 2001) Members of Malvaceae family are reportedly characterized with stellate, tufted stellate, tufted, 2- or 3-furcate or simple trichomes (Walas, 1959; Rutkowski, 2004). Celka et al., (2006) noted regular presence of unibranched unicellular and branched multicelllar trichomes in populations of Malca alcea, a member of Malvaceae family from Poland. The presence of two types of trichomes in U. lobata most likely reflects an evolutionary adaptation to produce secondary metabolites (in the glandular trichomes) and to ward off pests that attack the leaves (stellate trichomes). Perhaps this is a key to the various medicinal uses to which it is put by indigenous people. Anatomical features Transverse section of the petiole and stem revealed differences that may be associated with their functions. The epidermis of the petiole was uniseriate and glabrous while that of the stem was biserriate and covered with hairs (Plate 3 A &B). Cortical tissue in the petiole was multilayered than the case of the stem which had been reduced by the expanding wood formed by secondary growth. This is usual in woody herbaceous stems especially those that produce fibres. References
Baustista, L. M. A. 2000. Inquiry into the Antiflammatory activity of the syrup from the glycosides of the leave of (Urena lobata. Linn. Family: Malvaceae). Centro Escolar Universitario, Mandioko, Philippines. Celka, Z, Szkudlarz, P. and Bierenoj, U. 2006. Morphological variation of hairs in Malva alcea L. (Malvaceae). Biodiv. Res. Conserv. 3 4: 258 261. Cutler, D. F. 1978. Applied Plant Anatomy. Longman Inc, New York .103pp. Edeoga, H.O. and Ogbebor, N.O. 2001. Epidermal Features of some Nigerian species of Aneilema R, BR. (Commelinaceae). Journal of Economic & Taxonomic Botany. 19: 117 124.
Edeoga, H.O. and Ikem, C.I. 2001. Comparative morphology of leaf epidermis in three species of Boerhavia L. (Nyctaginaceae). Journal of Economic & Taxonomic Botany. 19: 197 205. FAO 1996. Nigeria: Country report to the FAO International Technical Conference On Plant Genetic Resources (Leipzig). Ed. Sarumi et al,. Ibadan, FAO. 2003. Food composition table for use in Africa. Food and Agriculture Organization. UN, Rome. http.//www.fao.org/docrep/003/x6877e/X687705.htm#ch5 FLEPPC, 1999. Invasive plant list (19 October 1999). Florida Exotic Pest Plant Council. Florida, USA. FRIM 2003. Plants Information. Urena lobata Griff, Pulut-pulut, Malvaceae. Forest Research Institute of Malaysia, Kuala Lumpur, Malaysia. Harris, P.J.C. 1986. Dormancy of Urena lobata L. Seeds. I. Development of Sulphuric Acid Scarification Techniques. Ghana Journal of Agricultural Sciences 14(19):79-84. Howes, O. A. 1979. In Useful and Everyday Plants and their Common Names. Longman Pub. 268pp. Hutchinson, J. and Dalziel, J. M. 1958. Flora of West Tropical Africa R. W. 2nd Ed. Vol. 1 Part II. Crown Agents for Oversea Government and Administrations. ISB. 2003. Atlas of Florida Vascular Plants, Institute of Systematic Botany, University of South Florida, Tampa, Fl. Irvine, F.R. 1963. West African Crops. Oxford University Press, Oxford. Johnsen, D.A. 1940. Plant Micro techniques. McGraw-Hill, New York, USA. Metcalfe C.R. & Chalk L .1950. Anatomy of the Dicotyledons. 2nd ed. Vol. 1: Systematic Anatomy of the Leaf and Stem, with a Brief History of the Subject. Clarendon Press, Oxford. Obute, G.C. and Omotayo, I.V. 1999. Micro- and Macro-morphological evidence for taxa separation in Hibiscus rosa-sinensis (L.). Feddes Repertorium 110 (3-4): 201 208. Ogunwenmo, K.O. and Ugborogho, R.E. 1999. Effects of chemical and mechanical scarification on seed germination of five species of Ipomoea L. Bol. Soc. Brot. 69: 147-162. Olowokudejo, J.D. 1990. Comparative morphology of leaf epidermis in the genus Annona (Annonaceae) in West Africa. Phytomorphology 40: 407 422. Okoli, B.E. 1987. Anatomical studies in the leaf and probract of Telfairia Hooker (Cucurbitaceae). Feddes Repertorium 98(3- 4): 231- 236. PlantNET 2009. Urena lobata L. National Herbarium of New South Wales © 1999 2009 Royal Botanic Gardens & Domain Trust, Sydney Australia. http://plantnet.rbgsyd.nsw.gov.au Purseglove, J. W. 1968. Tropical crops Dicotyledons. 2nd ed. Longman, Green and Co. London.. Rutkowski, L. 2004. Klucz do oznaczania roślin naczyniowych Polski niźowej. Wyd. 11, popr. I Unowocześnione, 814 pp. Wyd. Nauk. PWN, Warszawa. Stone, B. C. 1970. The flora of Guam. Micronesica 6:1-659. Wagner, W. L., Herbst, D. R. and Sohmer, S. H. 1999. Manual of the flowering plants of Hawaii. Revised edition. Bernice P. Bishop Museum Special Publication. University of Hawaii Press/Bishop Museum Press, Honolulu. 1919 pp. Walas, J. 1959. Malvaceae, Ślazowate. In: W. Szafer & B. Pawlowski (Eds.). Flora Polska. Rośliny naczyniowe Polski I ziem ościennych. 8, pp. 278-301. PWN Warszawa.
Table 1: Features of the branches of Urena lobata (N=35)
Type and number of branch
Table 2. Linear dimensions of mature and young leaf of different shapes (n=10)
Y= Young M = Mature
Table 3: Linear dimensions of petiole for different leaf shapes (cm) (n = 10)
Plate 1. Variations in leaf shape and margin exhibited in Urena lobata L. A. Deeply lobed lamina. B. Deeply palmately lobed margin. C. Simple ovate with serrate margin. D. Circular with shallowly depressed margin. E. Ovate shallowly lobed. F. Palmately shallowly lobed.
Plate 2. Photomicrographs of leaf trichome types in Urena lobata L. A. Abaxial leaf epidermis, notice the glandular trichome (arrowed) occurring alongside the variously armed stellate types. B. Adaxial leaf epidermis, notice the reniform glandular trichome (arrowed) alongside the stellate types. (x10 Mag.)
Plate 3. Transverse sections through the petiole and stem of Urena lobata. A. The petiole of showing epidermal, cortical and vascular bundle tissues. B. The stem showing biserriate epidermal tissue with hairs, cortical tissue and vascular tissue depicting secondary growth.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||