|
Ethnobotanical Leaflets 12: 413-418. 2008.
In Vitro Micropropagation of Talinum portulacifolium L. through Axillary Bud Culture
K. Thangavel1, M. Maridass2*, M. Sasikala3 and V. Ganesan4
1Department of Biotechnology, Sri Paramakalyani College, Alwarkurichi –627 412, Tamil Nadu 2Animal Health Research Unit, St. Xavier’s College (Autonomous), Palayamkottai, Tamil Nadu 3Dept. of Biotechnology, School of Life Sciences, Bharathidasan University, Trichy – 620 024, Tamil Nadu 4Asoka Trust for Research in Ecology and the Environment, Bangalore
*Corresponding Author: Email: [email protected]
Issued 22 June 2008 AbstractThe present study describes a protocol for rapid and large scale in vitro micropropagation of Talinum portulacifolium L. through axillary bud culture. The culture medium was optimized for propagation and high ex vitro survival rate was achieved. MS medium supplemented with 6µM BAP and 2µM IAA in combination was shown a better efficiency of shoot proliferation. Maximum of 8 new shoots were developed from a single explant (axillary bud) after 3 subcultures (subculture interval 15 days). Root development was facilitated by MS medium supplemented with both IBA (4µM) and NAA(1µM). Well rooted and partially acclimatized plantlets were transferred into poly cups for further acclimation. Key Words: Micropropagation, Axillary bud culture, Leafy Vegetables, Vitamin A Supplements, Talinum portulacifolium L.
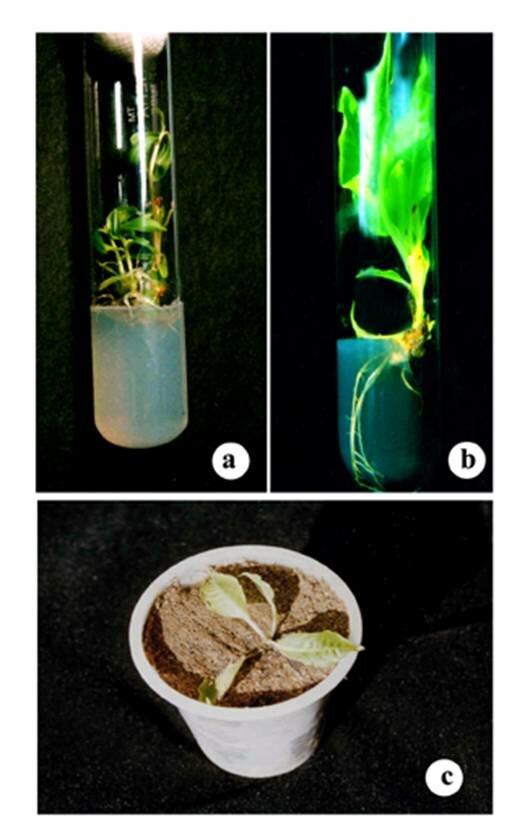
IntroductionTalinum portulacifolium L., an erect under-shrub belonging to the family Portulacaceae, is an important medicinal plant in the local system of medicine. It grows wild in Tamil Nadu, especially in the districts of Tirunelveli, Madurai and Thiruchirappalli (Nair & Henry, 1983). It is used as a green leafy vegetable due to its rich vitamin A and mineral content. The supplementation of the leaves of this plant is reported to be a better diet for strengthening the body. It is also used as a medicine for constipation and ulcer. A low survival rate by stem cuttings in Talinum portulacifolium L. restricts its mass propagation via conventional methods. Therefore, an efficient in vitro propagation system for producing this plant requires further studies on its potential medicinal values and germplasm evaluation and conservation. Gustavsson and Stanys (2000) reported that plants derived from tissue culture as having superior field performance to those derived from stem cuttings in terms of survival rate, fruit yield, rhizome production and total plant weight. In the present study the culture medium was optimized for propagation and high ex vitro survival rate was achieved. Materials and MethodsTalinum portulacifolium L. plants were collected from the natural habitat and maintained in our college nursery. Nodal explants with axillary buds were collected from the well established ones. Excised axillary buds were disinfected with 0.5% Sodium hypochlorite solution with a few drops of Teepol and washed thoroughly with tap water, then treated with 0.05% Mecuric chloride solution for 3 minutes and repeatedly washed with sterile distilled water under laminar flow chamber. Finally axillary buds were trimmed into appropriate size (0.5-1.0cm) and inoculated in the shoot induction medium. MS medium was supplemented with BAP and IAA in different concentrations individually and in combination and also with 2% sucrose (adjusted) and 0.8% agar. Each set of medium was maintained in triplicates. The PH of the medium was adjusted to 5.7 before autoclaving at 121ºC, with the pressure of 1.2 Kg/cm-2 for 15minutes. Cultures were maintained at 25 ± 2ºC under a 16/8 h (light/dark) photoperiod with light provided by cool-white fluorescent tube with an intensity of 80µmol/m-2s-1. All media formulations were tested to determine the extent to which they promoted shoot proliferation and development. Root induction medium (MS) was supplemented with IBA and NAA in different concentrations individually and in combination. All the observations were recorded and the average values were tabulated (Tables 1 and 2). Better results were photographed (Figures a-c). ResultsMedia formulation displayed a strong effect on the in vitro morphogenetic potential of the explants. Number of fresh shoots per axillary bud, length of shoots and their survival was influenced differently with different media combinations. Effect of PGRs on Shoot MultiplicationThe in vitro multiplication of shoots were strongly influenced by the cytokinin employed (BAP). MS medium supplemented with 6µM BAP and 2µM IAA in combination was shown a better efficiency of shoot proliferation. Maximum of 8 new shoots were developed from a single explant after 3 subcultures (Fig.a). After the multiplication, individual shoots were transferred into the fresh medium to improve the further withstanding capability. The fresh weight of the shoots increased proportionally to the rate by which it multiplied shoots in each medium (Table 1). Then the individual shoots were transferred into the root induction medium.
Effect of Auxins in Root DevelopmentProduction of adventitious roots differed significantly when different concentrations of auxins like NAA and IBA were supplemented with MS medium (Table 2). The primary roots became visible after 10 days of transfer. Shoots transferred into MS medium supplemented with both 4µM IBA and 1µM NAA produced even more roots, these 2-4cm long. More interestingly, a few number of roots attained their maximum length within 15- 20 days (Fig. b). For the further establishment of roots, plantlets were transferred into the poly cups filled with sterile garden soil and vermiculite soil mix in the ratio of 1:1 (Fig.c). During the first week of transfer, the plantlets were covered with a transparent plastic film to maintain high humidity and then fertilized at weekly intervals. The survival rate was examined 1 month after transfer and recorded as 60%. Discussion Recently, considerable attention has been devoted to medicinal herbs as a source of pharmaceutical components. As a parallel, improvement supplementation of leafy vegetables with nutritional and medicinal values are being stressed by the medical practitioners to improve strength and avoid vitamin and mineral deficiency problems. In this context, Talinum portulacifolium L. is being prescribed as one of the vital leafy vegetables due to its vitamin richness and curative values. The first step in the above two fields is to rapidly produce true-to-type and safe plants having a short life span (Mei-Chun Lu, 2005). The present study has successfully established a high frequency, mass propagation system for Talinum portulacifoluim L., a valuable leafy vegetable having known medicinal benefits. Murashige and Skoog (1962) medium has been designated for tissue culture of Tobacco and a wide variety of shrubs. In the present experiments, axillary buds cultured on different media combinations displayed significant differences in shoot proliferation rate and morphology. Earlier studies found BAP to be the most effective cytokinin for inducing shoot development (Lee and Wetzstein, 1990; Heloir et al., 1997). Heloir et al. (1997) reported that IBA provided a suitable auxin for in vitro rooting of Vitex vinifera. The observations of Herloi et al. (2000) coincide with the present study as the higher number of roots obtained in the MS medium supplemented with higher concentration of IBA than NAA. Mhatre et al. (2000) reported that IAA not only induces roots but also eliminated tufted shoots and calli in Vitis vinifera. The present report substantiates the earlier findings of Mhatre et al. (2000). MS medium supplemented with 2µM IAA along with BAP was able to yield maximum number of fresh shoots. ConclusionThe present study established the in vitro propagation system of Talinum portulacifolium L., a valuable leafy vegetable with high vitamin A and curative values for many ailments. High proliferation and survival rate was achieved with uniform and vigorous growth. This high proliferation could only be achieved through micropropagation rather than the traditional propagation methods. The regenerated plants did not exhibit any detectable variations in morphology or growth characteristics compared to their respective donor plants. The protocol described here could be used for large scale propagation of this medicinal plant.
AcknowledgementAuthors are thankful to the Principal, Secretary and Management of Sri Paramakalyani College, Alwarkurichi for their moral support and encouragement. The authors are also thankful to Dr.P. Ravichandaran, SPKCES, M.S. University, Alwarkurichi and Dr.M. Vivvekanandan and Dr.A. Ganapathy, Professors Department of Biotechnology, School of Life Sciences, Bharathidasan University, Trichy for their suggestions and constant guidance.
ReferencesBanerjee, N.P., DeLanghe, E.A.L., 1985. A tissue culture technique for rapid clonal propagation and storage of Musa (Banana and Plantains). Plant Cell Rep. 4, 351–354. Ford-Lloyd, B.V., Jackson, M.T., 1991. Biotechnology and methods of conservation of plant genetic resources. J. Biotechnol. 17, 247–256. Gustavsson, B.A., Stanys, V. 2000. Field Performance of “Sonna” lingonberry derived by Micropropagation versus stem cuttings. Hort. Science. Vol. 35: 742-744. Heloir, M.C., Fournioux, J.C., Oziol, L., Bessis, R., 1997. An Improved Procedure for the Propagationin vitro of Grapevine (Vitis vinifera cv Pinot noir) using Axillry bud microcuttings. Plant Cell Tissue Org. Cult. Vol. 49: 223-225. Lee, N., Wetzstein, H.Y., 1990.in vitro Propagation of Muscadine grape by Axillary shoot proliferation. J. Am. Soc. Hort. Sci. Vol.115: 324-329. Mei-Chun Lu. 2005. Micropropagation of Vitis thunbergi Sieb. Et Zucc., a Medicinal Herb through high frequency Shoot tip Culture. Scientia Horticulturae. Vol.107:64-69. Mhatre, M., Salunkhe, C.K., Rao, P.S., 2000. Micropropagation of Vitis vinifera L. towards an improved protocol. Sci. Hort. Vol. 84: 357-363. Murashige,T., Skoog, F., 1962. A Revised Medium for Rapid Growth and Bioassy with Tobacco Tissue Culture. Physiol. Plant. Vol.15: 473-497. Nair, N.C., Henry, A.N. 1983. Flora of Tamil Nadu. Series I: Analysis. (Botanical Survey of India). Vol.1: 24 Verpoorte, R., Van der Heijden, J.H.C. Hoge and H.J.G. ten Hoopen. 1994. Plant Cell Biotechnology for the Production of Secondary Metabolites. Pure & Appl. Chem., Vol.66. Nos. 10/11: 2307-2310. Abbreviations BAP- 6- Benzyle Amino Purine, MS Medium- Murashige and Skoog’s Medium(1962) NAA – Naphtyl Acetic Acid, IAA- Indole Acetic Acid, IBA- Indole Butyric Acid. |
|
S.No. |
Medium |
No. of Axillary Bud inoculated per vial |
No. of new shoots Developed |
Average Length of Shoots (mm) |
% of Response of Explant |
|
1 |
MS + BAP 1 µM |
1 |
2 |
12 |
32.0 |
|
2 |
MS + BAP 2 µM |
1 |
2 |
12 |
36.8 |
|
3 |
MS + BAP 3µM |
1 |
4 |
16 |
42.0 |
|
4 |
MS + BAP 4µM |
1 |
4 |
16 |
48.5 |
|
5 |
MS + BAP 5µM |
1 |
5 |
29 |
52.3 |
|
6 |
MS + BAP 6µM |
1 |
5 |
31 |
54.8 |
|
7 |
MS + BAP 7µM |
1 |
5 |
28 |
51.0 |
|
8 |
MS + BAP 8µM |
1 |
5 |
28 |
51.0 |
|
9 |
MS + BAP 9µM |
1 |
4 |
17 |
45.0 |
|
10 |
MS + BAP 10 µM |
1 |
4 |
17 |
45.5 |
|
11 |
MS + BAP 6 µM+ IAA 1µM |
1 |
6 |
32 |
58.0 |
|
12* |
MS + BAP 6 µM+ IAA 2µM |
1 |
8 |
41 |
69.5 |
|
13 |
MS + BAP 6 µM+ IAA 2µM |
1 |
6 |
33 |
68.0 |
|
14 |
MS + BAP 6 µM+ IAA 3µM |
1 |
5 |
29 |
60.0 |
|
15 |
MS + BAP 6 µM+ IAA 4µM |
1 |
5 |
26 |
55.8 |
* Media combination shown the best result (higher no. of new shoots)
Average of Values obtained from 10 vials.
Table 2. In Vitro Response of Shoots towards different combinations of Rooting medium.
|
S.No. |
Medium |
Root Induction |
Average Length of Roots (mm) |
% of Response |
|
1 |
MS + IBA 1µM |
+ |
3.0 |
31.0 |
|
2 |
MS + IBA 2µM |
+ |
8.0 |
33.0 |
|
3 |
MS + IBA 3µM |
++ |
13.0 |
37.0 |
|
4 |
MS + IBA 4µM |
++ |
20.0 |
39.0 |
|
5 |
MS + IBA 5µM |
++ |
17.0 |
36.0 |
|
6 |
MS + IBA 6µM |
++ |
16.0 |
34.0 |
|
7 |
MS + IBA 7µM |
++ |
16.0 |
32.0 |
|
8* |
MS + IBA 4µM + NAA 1µM |
+++ |
28.0 |
58.0 |
|
9 |
MS + IBA 4µM + NAA 2µM |
++ |
26.0 |
54.0 |
|
10 |
MS + IBA 4µM + NAA 3µM |
++ |
24.0 |
50.0 |
* Media combination shown better results in root development
- No Response + Response normal ++ Good response +++ Better results obtained
Average of values obtained from 10 vials.