|
Ethnobotanical Leaflets 13: 249-53. 2009.
Influence of Spatial and Temporal Variations on Phytoplankton Community Structure in Pachiparai Reservoir, Kanyakumari District, TN, India
P.J. Jepa Chandera Mohan1,2, S. Godwin Wesley1, S. Ramya2, N. Alaguchamy2, M. Kalayanasundaram2 and R. Jayakumararaj3
1 Department of Zoology, Scott Christian College, Nagercoil, KK Dist, TN, IN 2 Department of Zoology, Raja Doraisingam Government Arts College, Sivagangai – 630561, IN 3 Department of Botany, RD Government Arts College, Sivagangai – 630561, IN
Issued 30 January 2009
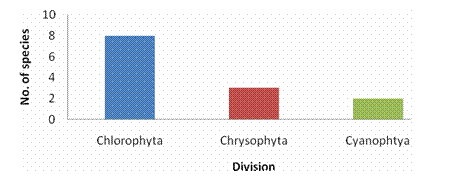
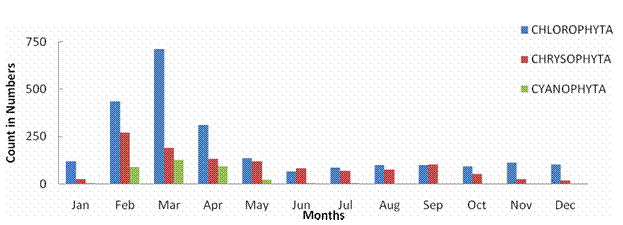
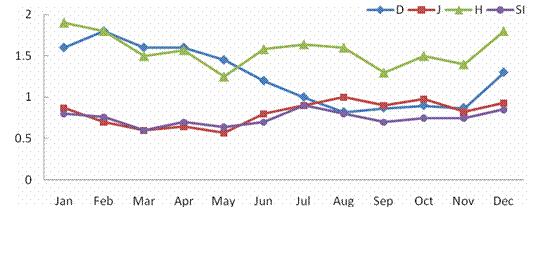
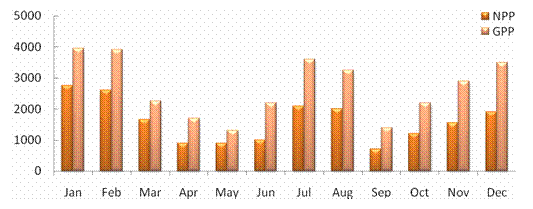
ABSTRACTPachiparai Reservoir in Kanyakumari District TN, India is influenced by seasonally reversing monsoon and the characteristics of the water body influenced by riverine inflow. An effort to elucidate the spatial and temporal variation in the phytoplankton community was carried out. The observations were primarily made by during Jan 2004 – Dec 2004. Average phytoplankton abundance and primary production varied marginally during the period of study. Surface phytoplankton population showed seasonal variations and the abundance peaked during the beginning of North East monsoon (November). The inflow of water during the monsoon into the reservoir was the prime factor that influenced input of the nutrients and appeared to enhance the primary production. This study provides the details of the spatial and temporal variation and explores the implication and causative factors responsible for the dynamics of the phytoplankton populations. KEYWORDS: Pachiparai Reservoir; Primary Production; Phytoplankton Diversity; Shannon – Weiner diversity index; Sympson index.INTRODUCTIONA number of factors have been attributed to influence the diversity of fauna and flora in any aquatic ecosystem. An assessment of biological productivity of any aquatic ecosystem is essential to ascertain whether the productivity is sufficient enough to support the standing stock of commercially important fishes. Topical reservoirs are generally characterized by rich population of Phytoplankton (Srinivasan, 1964). Among the various factors that influence the diversity of fauna and flora of a reservoir ecosystem, phytoplankton play a pivotal role in the production of organic matter, which in tern is decided by the various environmental factors. Studies on the primary productivity in Indian lentic systems are meager (Srinivasan, 1964; Joshi et al., 1995; Sukumaran and Dhas 2001; Shrivastava, 2005). In the present investigation the primary productivity and phytoplankton diversity in Pachiparai Reservoir in Kanayakumari District TN, India was assessed to provide the present status of primary productivity and phytoplankton diversity of reservoirs in South India. MATERIALS AND METHODSThe investigation was carried out over a period of twelve months Jan 2004 to Dec 2004. Phytoplankton samples were collected by filtering the water through bolting silk cloth (No. 25) and preserved in 4% formalin. The systematic identification of the phytoplankton was done adopting the standard keys of Desikachary (1959) and Cramer (1984). The enumeration of phytoplankton count was carried out with the help of a haemocytometer. The species diversity indices, viz., Shennon – Weiner diversity index (H1), Sympson index (SI) and evenness index (D) were computed. Assessment of primary productivity was done as per the procedures of Apha (1992). RESULTS AND DISCUSSIONIn the present study, from the Pachiparai reservoir at least 13 species of phytoplankton were recorded. Off these 8 species belong to the division Chlorophyta, 3 Chrysophyta and 2 Cyanophtya (Fig. 1). The division Chlorophyta was comprised of Staurastum longipes and S. fremantii which were recorded almost through the course of investigation. Staurodesms sp. did not occur during Apr and may and Ulothrix sp. from Dec to Apr. The division Chrysophyta, was mostly represented by Melosira sp. during Jan - Apr, Naviculata sp. Jun - Mar and Botryococcus sp. Feb - Nov. In the case of Cyanophyta, Oscillatoria sp. occurred in abundance from Feb - May and Microcystis sp. from Jan – Jul (Fig. 2). In the study period maximum phytoplankton density (1021 Nos-1) was recorder in the month of Nov. Stauastum sp. where recorded through the year, where as Zygnema sp. was the most abundant species (602 No-1) recorded in the month of Mar (Table1). The species diversity H1 ranged from 1.242 (Sep) to 1.897 (Jan), SI from 0.614 (May) 0.898 (Jul) and J1 from 0.646 (Apr) to 956 (Aug) (Fig. 3).The primary productivity in the planktonic algae is maximum in the upper photic zone of the water body. Therefore, assessments of the standing crop and rate of production are important factors that determine the fishery potential (Salakar and Yearagi, 2004). In the present study, primary production varied between 1412 mg C m-3d-1 (Sep) to 3902 mg C m-3d-1 (Jan). The high values of net primary production (NPP) gross primary production (GPP) could be recorded during the months of Jan, Feb, Jul, Aug and Dec all these months being the post monsoon period (Fig. 4). The seasonal abundance of phytoplankton in Pachiparai reservoir was very high during post secondary monsoon season (Jan to Mar) than the post primary monsoon season (Aug and Sep). The phytoplankton population was considerably low during period of high precipitation; this could be due the high turbid nature of the water in the reservoir which in turn brings about the decline in the intensity of light that pears though the water on the upper region (Sugunan, 1980). A direct relationship between monsoon flow and plankton density has been reported in Richand reservoir (Nataraj, 1976). The Shennon – Weiner diversity index (H1) and Sympson index (SI) of the reservoir increased due to varied proliferation of phytoplankton during the two post monsoon seasons. This observation is in agreement with the previous report of Sugunam (1991) for net plankton in Nagarjunasargar reservoir. Sukumaran and Karthikeyan (1999) recorded similar observations in the species diversity of peri-phyton in Markonahalli reservoir. Decline in the values of diversity indices in the southwest monsoon and northeast monsoon coincide with the decline of species index of the phytoplankton community. This observation is in accordance with that of Margalef (1963). The establishment of some species during certain months during certain months could be attributed to the prevalence of favorable environmental conditions. Evenness index j was higher in certain months apparently due to comparable distribution of various genera and the species in the habitat as affirmed Whilm and Dorris (1968). Phytoplankton synthesis new organic carbon by the process of photosynthesis in the present study the values of gross primary productivity and net primary productivity were higher in the post monsoon seasons. Subbamma (1993) observed that the population of the phytoplankton was much higher in the summer than in other seasons. This could be due to the prolonged day length and high intensity of light during this period. Srinivasan (1964) observed similar results in the Ammaravathi reservoir. In the present study the maximum value of NPP was recorded in the post monsoon period. This could be due to the prolonged availability of light and inflow of nutrients during the monsoon. ACKNOWLEDGEMENTSThe authors are thankful to Principal, RDGAC and Management of Scott Christian College for their constant support and encouragements. The authors express their gratitude to Prof. G Muthiah, Head Department of Zoology, RDGAC for valuable comments and suggestions during the preparation of this manuscript. REFERENCES1) Apha (1992). Standard method for the examination of water and waste water. 18th Ed. Washington DC. 2) Desikachary K (1959). Cyanophyta ICAR Publication. New Delhi, India. 3) Joshi BD, Bisht RCS and Samual VP (1995). Primary productivity in Western Ganga cannel at Howrah Indian Journal of Ecology 21(2):123-126. 4) Margalef R (1963). On certain unifying principles in ecology. An Nat 97:357-374. 5) Nataraj VV (1976). Fish farming in man made lakes. Indian farming 26(6):24-33. 6) Salaskar PB and Yeragi SG (2004). Primary productivity in Powai lake, Mumbai, Maharashtra. Journal of Aquatic Biology.19 (1):19-22. 7) Singh DN and Das AK (2002). Spatial-temporal distribution of phytoplankton in relation to physio-chemical features in peninsular lake. Proceeding National Academy of Science, India. 72(3-4):293-303. 8) Srinivasan A (1964). Hydrological study of a tropical impoundment Bhavanisagar reservoir, Madras state, India, for the year 1950-61, Hydrobiologia 54:514-539. 9) Srivastava NP (2005). Plankton status of Ravisankarsagar reservoir. Journal of Inland fish Society, India. 37(2):43-47. 10) Subbamma DV (1993). Primary production in temple pond and a fish pond in AndraPradesh, India. Journal of Aquatic Biology 18(142):7-9. 11) Sugunan VV (1991). Changes in the phytoplankton species diversity index due to artificial impoundment in river Kroshma at Nagarjunasagar. Journal of Inland Fish Society, India. 23(1):64-74. 12) Sukumaran PK and Karthikeyan M (1999). Seasonal abundance and species diversity of periphyton in Markonahalli reservoir, Karnataka Journal of Inland Fish Society, India 31(2):93-98. 13) Sukumaran PK and Das AK (2001). Distribution of phytoplankton in some fresh water reservoir Karnataka. Journal of Inland Fish Society, India. 33(2):29-36. 14) Whilm JR and Dorris TC (1968). Biological parameters of water quality. Biosciences 18:447-481. Table 1. Phytoplankton Diversity in the Pachiparai Reservoir.
Fig. 1. Divisions of Phytoplanktons in Pechiparai Resevoir. |