|
Ethnobotanical Leaflets 12: 204-216. 2008.
Floristic Inventory and Quantitative Vegetation Analysis of Tropical Dry Deciduous Forest in Piranmalai Forest, Eastern Ghats, Tamil Nadu, India
Pitchairamu C1, Muthuchelian K2 and Siva N2
1Department of Botany, P.T.M.T.M. College, Kamuthi- 623 604, Ramanathapuram District, TamilNadu, India 2Centre for Biodiversity and Forest studies School of Energy Sciences, Madurai Kamaraj University, Madurai 625 021, TamilNadu, India Issued 18 April 2008 ABSTRACT Tree diversity, species richness, basal area, population structure and distribution patterns were investigated in disturbed, moderately disturbed and undisturbed areas of the tropical dry deciduous forest of Piranmalai, Eastern Ghats in Tamil Nadu. The forest areas were selected based on the disturbance index. Six sites of 0.1ha area were established in the Piranmalai forest. Two sites, Nehru park (N.P) and Foot hill (F.H), were located close to the mining, quarrying and human settlement area, while two other sites, Vannar iruppu (V.I) and Alaguchokkan (A.C), are located in a selective felling area, and two others, Dhargha area (D.A) and Veerappan koil (V.K), are situated in a relatively undisturbed forest. These are 3 to 5 K.m apart in the forest disturbed stand (60% disturbance index), Moderately Disturbed stand (30% disturbance index), and Undisturbed stand (10% disturbance index) . Tree species richness varied along the disturbance gradient in different stands. The undisturbed stand showed the highest species richness (11 9). Species richness was lowest (5-4) in the Disturbed stand, while in the Moderately Disturbed stand the diversity was somewhat higher (8 7). The Shannon Wiener index for tree species ranged from 1.33 to 2.184 in all the stands. The highest tree diversity was recorded in the undisturbed stand and the lowest in the Disturbed stand. The stands differed with respect to the tree species composition at the family and generic level. Key words: Eastern Ghats, Piranmalai, Disturbance, Tropical dry deciduous forest.
INTRODUCTION Tropical forests occupy ca 7% of the earths area (Myers, 1984). In India, they occupy ca 84% of the total forest cover (637293 Km2), which is 19.39% of the total geographical area (State Forest Report, 1999). Though the total geographical area of tropical wet evergreen forests is ca 15010 Km2 (10.7% of the tropical forest cover of India), phytogeographically these forests are rich in biological diversity (Chandrasekharan, 1960). These forests face a serious threat, both natural as well as anthropogenic. Eventually, several species have become endangered. This implies a poor natural regeneration potential of the species. Thus, the need to set priorities for conservation of diversity has become inevitable. Identification of conservation areas ideally requires exhaustive knowledge of species and ecosystem diversity and distribution (Menon et al., 2001, Angelstan et al, 2004 a, Felix et al, 2004 and Reese et al., 2003). Primary forests of Asia, particularly those of the Western Ghats and the Eastern Ghats of Peninsular India are disappearing at an alarming rate due to anthropogenic activities and are replaced by forests comprising inferior species or their land use pattern changed (Parthasarthy, 1999). The disappearance of tropical forests threatens us at a time when our knowledge on their structure and dynamics is woefully inadequate (Hubbell and Foster, 1992). Understanding of forest processes is necessary for assessment of potential impacts, the amelioration of effects of disturbance, optimisation of productivity and rehabilitation of ecosystem (Congdon and Herbohn, 1993). As there is so much species diversity, tropical trees are especially interesting subjects (Condit et al., 1996) and species diversity is generated by species interaction such as competition and niche diversification (Pianka 1966; Bada 1984), the latter being greatly manifested in the tropics due to high humidity and temperature (Ojo and Ola-Adams 1996). Quantitative plant biodiversity inventories of Indian tropical forests are available from various forests of Western Ghats (Sukumar et al., 1992; Ganesh et al., 1996; Pascal and Pelissier, 1996; Ghate et al., 1998; Parthasarathy, 1999; Parthasarathy and Karthikeyan, 1997a; Ayyappan and Parthasarathy, 1999) and on the Coromandal coast (Parthasarathy and Karthikeyan, 1997; Parthasarathy and Sethi, 1997), but the Eastern Ghats is poorly studied in these aspects, except those of Kadavul and Parthasarathy (1999a) in the Shervarayan hills and Kadavul and Parthasarathy (1999b) in the Kalrayan hills. Disturbance to an ecosystem means any discrete event that disrupts the ecosystem, community or population structure, or the physical environment (Pickett & White 1985). Species composition, community dynamics and human welfare services of forest ecosystems become adversely affected by disturbances of both natural and anthropogenic origin (Sousa 1984). Whitemore & Burslem (1996) classified disturbance into large scale or community wide (landslides, volcanoes, drought, lighting, forest fire and various human activities) and small-scale disturbances such as mortality of few trees. In fact many kinds of disturbances both natural and anthropogenic are amenable to scientific experimentation and immeasurable directly. Most of the studies on forest ecosystems in relation to disturbance were focused on species-rich tropical rain forests (Ashton 1993; Aravind et al. 2001; Bhuyan et al. 2001; Whitemore & Burslem 1996) or temperate forests (Gilliam 2002; Schumann et al. 2003). Dry deciduous forests of tropical areas are under constant disturbances of both climatic and anthropogenic origin. In India, habitat destruction, over exploitation, environmental pollution and anthropogenic pressure are the major disturbances to forest ecosystems (UNEP 2001). The dry tropical forest accounts for 38.2% of the total forest cover of India (MoEF 1999), which is largely disturbed by lopping, burning, overgrazing and clearing for cultivation. Such disturbances lead to their conversion into species-poor forest ecosystems. Habitat destruction is the leading cause of species extinction and biodiversity loss in natural ecosystems (Koh et al. 2004; Pimm & Raven 2000). Unfortunately, studies on tropical dry deciduous forests in relation to disturbances were much limited in India (Khera et al. 2001; Puyravaud et al. 1995) particularly, on Eastern Ghats in south India (Rajan et al. 1995). Thus, the present study evaluated the disturbance to a tropical dry deciduous forest of the Piranmalai forest (Eastern Ghats), Sivagangai, south India using selected direct (number of trees, number of butterflies and herb density) and indirect (number of sunspots and number of man-made tracks) measures.
MATERIALS AND METHODS
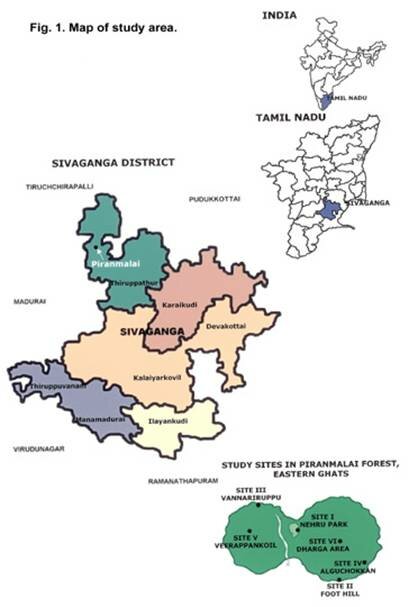
This research was conducted in the Piranmalai forest of the Eastern Ghats in Tamil Nadu. It was one of the major hill ranges in the southeastern part of the Eastern Ghats located in Sivaganga district of TamilNadu, between North latitude 9o 31' to 10o 27', East longitude 78o 8' to 79o 2 (Fig.1) the elevation of the hill measures about 2450 feet above the sea level. A bridal path runs from the foot of the hill to top. The district as a whole was very dry and had a hot tropical climate. April, May and June are the hottest months. The South west monsoon brings rainfall from September to November. From December to February rainfall is received from the North east monsoon. (Fig.2) The district has a mean annual rainfall of 1176.83mm. The taxonomy of the soil was fine loamy, Koolinitic, isohyperthermic, calcareous and very deep.
The vegetation varies considerably with altitude. The food hills harbour scrub vegetation which extends up to an altitude of 400M, dry and mixed deciduous forest occur in the whole hill areas. These forests have been free from human interference over the centuries. This has been mainly due to the very limited human population in the area and the location of the villages. Drastic extraction of Medicinal plants, timber and honey collection were taken up from the 18th century. Some fellings in this area started in 1969, mostly for opening up roads, Annona squamosa were planted after clearing sub canopy with the object to cultivate a suitable cash crop that can yield revenue to the area people.
Six 0.1 ha quadrate plots (N.P, F.H, A.C, V.I, V.K and D.A) are established. Sites N.P and F.H are located near human settlements, besides the road. The site N.P is closed to Oduvanpatty village, F.H is near to Piranmalai village human activities are more. They are always collecting forest products daily. Human activities in the area include selective logging of trees such as Albizia amara, Wrightia tinctoria, Alangium salvifolium for fuel and for making agricultural implements. These sites are closed to the agricultural field and stone mining areas. Sites AC and V.I are situated in the interior region. Illegal selective felling for fuel wood and medicinal plants collecting was witnessed on two sites. Sites V.K. and D.A are situated on the peak at an altitude, they are steeper than the other sites and are less disturbed due to poor accessibility, the forest floor contains huge boulders. Occasional browsing and by cattle was noticed.
Phytosociological studies were conducted in all the six study sites during 2002-2003. The density, frequency and basal area were estimated in the randomly placed quadrates. 10 quadrates (10 x 10 m) for trees (individuals with gbh more than 30.1 cm) seedlings and saplings of trees were constructed. Girth at breast height (gbh) at 1.37 m above ground level of all trees and tree seedlings in each quadrate was measured for each species. Diameter at ground level of seedlings was measured using Vernier calipers. Epiphytes were not sampled in this study. Plant species were identified in the field with the help of Gamble (1925) and Mathew (1981-1988) and later counter checked with the reference materials available at the Madras Herbarium (Botanical Survey of India), Coimbatore. A reference collection of specimens were submitted to centre for Biodiversity and Forest studies, School of Energy sciences, Madurai Kamaraj University, at Madurai, in India. The vegetation data were analyses for relative frequency, relative density and relative dominance. The sum of relative frequency, relative density and relative dominance represented the Importance Value Index (IVI) for various species (Curtis, 1959). The index of the dominance of the community was calculated by Simpsons index (Simpson, 1949) as C = S (ni/N)2 The index of species richness (d) was calculated following Menhinick (1964).D = s/ึn. The evenness index of the community (e) was calculated following Pielou (1966) as e = H/log S. Index of similarity (s) between two samples was calculated following Odum (1971) as S = 2c/a+b RESULTS AND DISCUSSION
Tree species richness A total number of 16 tree species belonging to 14 genera, 16 species and 12 families was recorded from six sites (disturbed, moderately disturbed and undisturbed) of study area.
Tree species richness varied according to the disturbance gradient in the different stands. Consolidated data of phytosociological studies were given in (Table 1). Tree species richness (number of tree species) was higher in site VI stand (11 species) followed by site V stand (9 species). Site V stand was found to have greater density (620 ha-1) and basal area (48.494 M2 ha-1). However, greater diversity index 2.184 and species richness (1.556) were recorded in site VI stand. Dominance index (0.301) in site III and evenness index (2.164) in site II stand were found to be greater. Number of species (4), diversity index (1.303), density (220 ha-1) and species richness (0.853) were least in site II stand. However, basal area (6.587 M2 ha-1) and evenness index (1.932) in site I stand and dominance index (0.135) in site VI stand were found to be least. The highest tree species diversity was recorded in the sites VI, V, IV, III stands and lowest in the sites II and I stands. The similarity index value was maximum in the site V, VI stands and minimum in the sites I, II stands (Table 2). The species richness of 16 tree species in the 0.6 ha of the tropical dry deciduous forest in Piranmalai forest of Eastern Ghats reflects its low diversity status. As all the six study sites are located within a distance of 6 km and an elevation between 550 and 1100 m, soil, climate and topography largely remain the same. But the difference in human interference between each study site has considerable influence on species richness, from 4 species ha-1 in the site II stand to 11 species ha-1 in the site VI stand. The diminishing tree diversity along elevational gradients in Piranmalai forest is in conformity with (Lieberman et al.,1996) and (Heaney and Proctor,1990). The present forest study sites species richness (4-11) was lowered when compared to that of semi-evergreen forests of Indian Eastern Ghats and Western Ghats, ie the number of the species in Kolli hills (26-56; Chittibabu and Parthasarathy, 2000a), Kalrayan hills (42-47; Kadavul and Parthasarathy, 1999a), Shervarayan hills (33-50, Kadavul and Parthasarathy, 1999b), three sacred groves of Kerala (14-23; Chandrashekara and Sankar, 1998), Thirumanikuzhi sacred grove (38, Parthasarathy and Karthikeyan, 1997), Kuzanthaukuppam sacred grove (42; Parthasarathy and Karthikeyan, 1997) and also with Peninsular India, species richness was lowered when compared to that of various sites of western Ghats ranging from 30 species ha-1 Nelliampathy (Chandrashekara and Ramakrishnan, 1994) to 57 species ha-1 in Mylodai and Courtallam reserve forest (Parthasarathy and Karthikeyan 1997), 64 to 82 species ha-1 in the medium elevation evergreen forests at Kalakad (Parthasarathy 1999), 90 species on a 3.82 ha-1 scale in Kalakad Mundanthurai tiger reserve forest (Ganesh et al., 1996) 48 to 74 species in the humid tropical forest in Tamil Nadu (Swamy et al., 2000), a range of 52 to 79 species ha-1 in the 30 ha-1 of tropical evergreen forest, Varagaliar, Anamalis (Ayyappan and Parthasarathy, 1999), 30 to 31 species in the tropical dry evergreen forest on the Coromandel coast (Venkateswaran and Parthasarathy, 2003) 87 species in Sal forest of Eastern Himalayas (Umashankar, 2001), 13-21 species Sal forest of Central Himalayas Doon valley (Pande, 1999), 3-20 species in Corbett National Park (Singh et al., 1995). 16 to 54 species in tropical wet evergreen forest in Arunachal Pradesh, Eastern Himalayas (Putul Bhuyan et al., 2003), 123 species in Jaintia hills in Meghalaya, north east (Upadhaya et al., 2003), 11 to 150 species in the Tansa valley (Radha Veach et al., 2003), 132 to 192 species in the sacred grove in Meghalaya, north east India (Mishra et al., 2004), 208 species in the forest of Gorakupur (Pandey et al., 2003) Dominance of tree species Dominance, calculated as the IVI of different species varied greatly in different stands. The IVI of trees in the study area was given in (Table 2). Greater number of tree species (11) were recorded in site VI stand followed by site V stand whereas least number of species (4) were seen in site II stand. Albizia amara was found as the dominant tree species in all the study sites except in site IV stand, whereas Albizia lebbeck dominated in site IV, Lepisanthes tetraphylla in site III stand, Diospyros montana in site V and VI stands, Holoptelia integrifolia in site I and IV stands and Pleiospermium alatum in site IV stand were the least dominant in terms of IVI. The dominance index in the present study ranged from 0.135 to 0.311 which is comparatively higher than that of Courtallum (0.079; Parthasarathy and Karthikeyan 1997), Thirumanikuzhi (0.1251; Parthasarathy and Karthikeyan, 1997), Kuzhanthanikuppam (0.173; Parthasarathy and Karthikeyan, 1997) and Nelliampathy (0.08; Chandrashekara and Ramakrishan, 1994). However, the values of dominance were closer to those of the three sacred groves of Kerala (0.12-0.36; Chandrashekara and Sankar, 1998) but lower than that of Puthupet (0.15-0.40; Parthasarathy and sethi, 1997) and Kolli hills (0.366-0.83; Lakshmi, 1995). Tree families, genera and species Enumeration of plant families, genera and species in different stands showed the presence of 8 families with 8 genera in site V stand, 9 families with 10 genera in site VI stand, 7 families with 7 genera in site III stand, 7 families with 8 genera in site IV stand and 5 families with 5 genera in site I stand 4 families with 4 genera in site II stand (Table 3).
Out of the 8 families in site V stand were represented by a single genus, 9 families in site VI stand, one was by more than one genus and eight were by a single genus, 7 families with 7 genera in site III and IV stands, 5 families with five genera in site I stand and 4 families with four genera in site II stand.
Mimosaceae was the densest tree family (47.03%) in the forest stand followed by Rubiaceae (11.86%), Apocyanaceae (9.88%), Alangiaceae (7.51%), Loganiaceae (7.11%), Herandiaceae (4.74%), Ulmaceae (3.95%), Sapindaceae (3.56%), Rutaceae (1.91%), Ebenaceae (1.58%), Fabaceae (1.19%) and Meliaceae (0.40%).
Tree density and species richness in different girth classTree stand density and species richness consistently decreased with increasing Girth class of tree species from 30 to > 240 cm gbh Girth (Fig. 2.1 and Table 2.4). The highest tree stand density and species richness were recorded in the girth class 30-60 and 60-90 cm gbh in all stands. In the site V and VI stands the highest tree stand density and species richness were found in the 90-120, 120-180 and 180-210 gbh cm Girth class, while in the site V stand the 210-240 cm gbh Girth range was recorded. In the site I and II stands no tree was recorded of more than 120 cm gbh Girth. The contribution of lower size class trees (30-60 cm gbh) to species richness ranged from in site V stand (22.22%) to in site II stand (100%) and (60-90 cm gbh) to species richness ranged from in site I stand (40%) to site VI stand (81.82%). In medium Girth class (90-120 cm gbh) to species richness ranged from in site I stand (20%) to in site V stand (77.78%) and (120-150 cm gbh) to species ranged from in site IV stand (12%) to in site V stand (66.67%). In the highest girth classes (180-210 cm gbh) and (210-240 cm gbh) species richness was found in sites IV and V stands. In site I and II stands no tree species richness was recorded of more than 120 cm gbh.
The contribution of lower size class trees (30-60 cm gbh) to density ranged from in site V stand (17.74%) to in site I stand (65.23%) and (60-90 cm gbh) to density ranged from in site IV stand (18.64%) to in site VI stand (38%). In medium girth class (90-120 cm gbh) species density ranged from in site I stand (8.70%) to in site V stand (24.19%) and (120-150 cm gbh) to density ranged from in site III stand (10.8%) to in site V stand (25.81%). In the highest girth classes (150-180 cm gbh), (180-210 cm gbh) and (210-240 cm gbh) density were recorded in sites IV and VI stands. In site I and II stands no tree density was found more than 120 cm gbh (Fig. 3 and 4 and Table 4).
Mimosaceae is the most important (47.03%) individualised family in the Piranmalai forest study area. This feature of a single family dominance with 47.03% contribution is greater in the Shervarayan hills, the other Indian Eastern Ghats site, where the family Oleaceae (26.6%) dominated (Kadavul and Parthasarathy 1999a) and Jengka forest reserve, Malaysia, where the Euphorbiaceae with 24.6% dominated (Ho et al., 1987).
Mimosaceae, Rubiaceae, Apocyanceae, Alangiaceae and Loganiaceae were the most specious families in the tropical dry deciduous forest of Piranmalai, while in the adjacent Shervarayan hills Euphorbiaceae and Rubiaceae were most dominant (Kadavul and Parthasarathy 1999a). Broad leaved forests in Taiwan showed Lauraceae and Rubiaceae to be dominant (Hara et al., 1997).
In central Amazonian upland forests, Leguminoasae, Lauraceae, Sapotaceae, Chrysobalanceae and Moraceae were the richest families (Ferreira and Prance, 1998).
The family dominance changed from the sites I, II stands to sites III, IV stands and to sites V, VI stands the change being more conspicuous in the sites I and II stands. A similar result has also been reported by (Thorington et al., 1982), (Parthasarathy and Karthikeyan, 1997) and (Parthasarathy and Sethi, 1997). The drastic variation in tree species composition and abundance among the six sites, is evidently due to anthropogenic activities, especially in sites I and II stands. The trend of decreasing diversity and density with increasing Girth class was in conformity with the studies of (Hara et al., 1997), (Jeffre and Veillon, 1990), (Kadavul and Parthasarathy, 1999a), (Newbery et al., 1992) and (Paijmans, 1970). In the present study sites IV and V stands, matured stands with good regeneration were reported from Jenka forest reserve, Malaysia (Poore 1968; Ho et al., 1987), Costa Rica (Lieberman et al., 1985), Brazilian Amazon (Swaine et al., 1987; Campbell et al., 1992), Sungei Menyala in Malaysia (Manokaran and Kochummen, 1987), Mudumalai in India (Sukumar et al., 1992) and in Monteverda, Costa Rica (Nadkarni et al., 1995). Sites I and II stands respectively with had low density in smaller and larger girth classes. This corroborates our field observation during this investigation. In site I and II stands, smaller Girth class trees were easy to cut and transport as head loads since the sites were closer to union road and human settlements. Majority of the cut trees were recorded in first and second Girth classes ie. (30-60 and 60-90 cm gbh). These findings indicated the preference of lower Girth class wood by the villagers to meet the household fuel wood demand and also the demand of poles for construction purposes. CONCLUSION Biodiversity is essential for human survival and economic well-being and for the ecosystem function and stability. The biodiversity inventories of Piranmalai forests particularly those of the poor known tropical dry deciduous forest in Piranmalai forest of Eastern Ghats. Our results suggest that a history of disturbance, accelerated during the last century has reduced biomass, canopy height and tree density in forests. The forest and soils have thus retained less of the precipitation of the monsoon and increased the effect of the post monsoon dry season on the vegetation. The quantitative biodiversity data of Eastern Ghats will be useful in forest management and conservation. Forest has been cleared for park construction, road construction, tourist resorts and agricultural land encroachments. In recent years it has become increasingly apparent that conservation of tropical forest cannot rely solely on protected area networks. A comprehensive approach to forest conservation must therefore incorporate the sustainable management of land outside protected areas (Boyle, 1977) and this requires an understanding of how human activities impact on forest resources. In the Piranmalai forest sites this is essential to prevent further expansion of construction and plantation area, as the protection of existing forests here is crucial for biological conservation of the species. Occasionally wild animals such as peacock, rabbit and monkey are hunted. Trees are selectively felled for firewood, household, furniture and fencing and for making minor agricultural implements. An alternative fuel energy source for cooking needs to be provided for the 8000 inhabitants of the hill area in order to prevent felling, and hotels in this tourist area should be instructed to use cooking gas as to save the hill forest. Presently, there is a need for increased legal protection, well designed management practices and intensive afforestation at selected altitudes especially foot- and mid-hill areas for the sustainable utilization of the dry deciduous forests.
Table 1. Consolidated details of Phytosociological analysis in the tropical dry deciduous forest ecosystem at Piranmalai forest, Eastern Ghats, Tamil Nadu, India.
Table 2. Importance value indices (ivi) of different trees in the tropical dry deciduous forest ecosystem at Piranmalai forest, Eastern Ghats, Tamil Nadu, India.
Table 3. Family wise contribution of genera(G), species(S) and density of trees in the tropical dry deciduous forest ecosystem at Piranmalai forest, Eastern Ghats, Tamil Nadu, India.
Table 4. Species richness (No. of species), and density/ha of the trees (10-30 cm dbh) recorded at Piranmalai forest, Eastern Ghats, Tamil Nadu, India.
REFERENCES: Angelstam, P., Donz Brcuss, M. & Roberge, J.M., (Eds) (2004a). Targets and tools for the maintenance of forest bio-diversity. Ecological Bulletins, 51. Aravind, N.A., D. Rao, G. Vanaraj, J. Poulsen, R. Uma Shankar & K.N. Ganeshaiah. 2001. Anthropogenic pressure in a tropical forest ecosystem in western ghats, India: are they sustainable? pp. 125-128. In: K.N. Ganeshaiah, R. Uma Shankar & K.S. Bawa (eds.) Tropical Ecosystems: Structure, Diversity and Human Welfare. Oxford & IBH, New Delhi. Ashton, P.S. 1993. The community ecology of Asian rain forests in relation to catastrophic events. Journal of Biosciences 18: 501-514. Ayyappan, N. and Parthasarathy, N., 1999. Biodiversity inventory of trees in a large scale permanent plot of tropical evergreen forest at Varagalaiar, Anamalais, Western Ghats, India. Biodiversity and conservation. 8: 1533-1554. Bhuyan, P., M.L. Khan & R.S. Tripathi. 2001. Tree diversity and population structure in undisturbed and human impacted tropical wet evergreen forests of Arunachal Pradesh, northeast India. pp. 114-115. In: K.N. Ganeshaiah, R. Uma Shankar & K.S. Bawa (eds.) Tropical Ecosystems: Structure, Diversity and Human Welfare. Oxford & IBH, New Delhi. Campbell, D.G., Stone, J.L. and Rosas Jr, A., (1992) A Comparison of the Phytosociology and dynamics of three flood plain (Varzee) forest of known ages, Rio Jurua, Western Brazilian Amazon. Bot.J. Linn. Soc. 108, 213 37. Chandrasekaran, U.M., and Ramakrishnan, P.S., 1994. Vegetation and gap dynamics of a tropical wet evergreen forest in the Western Ghats of Kerala, India. J. Trop. Ecol. 10: 337-354. Chandrasekharan C., 1960. Forest types of Kerala state. Special paper submitted for Diploma in Forestry. New Forest, Dehra Dum, India. Chandrashekara, U.M., and Sankar, S., 1998. Ecology and Management of sacred grove in Kerala, India. Forest Ecology and Management. 112: 165-177. Chittibabu, C.V. and Parathasarathy, N., 2000a. Attenuated tree species diversity in human-impacted tropical evergreen forest sites in Kolli hills, Eastern Ghats, India. Biodiv. Conserv. 9: 1493-1519. Congdon RA., and Herbohn JL., (1993) Ecosystem dynamics of disturbed and undisturbed sites in north Queenland wet tropical rain forest I Floristic composition , climate and soil chemistry . Journal of Tropical Ecology 9: 349 369. Felix, A.B., Campa, H. III, Millenbah, K.F., Winterstain, S.R.& Moritz, W.E. (2004). Development of landscape. scale habitat-potentiakl models for forest wildlife planning and management wild life society Bullletin , 32, 795 806. Gamble, J.S., 1925. Flora of the Presidency of Madras. Adlard and Son, London. Volume 1-3, 2017 pp. Ganesh, T., Ganesan, R., Soubadradevy, M., Davidar, P. and Bawa, K.S., 1996. Assessment of plant biodiversity at a mid-elevation evergreen forest of Kalakad Mundanthurai Tiger reserve, Western ghats, India. Curr. Sci. 71: 379-392. Ghate, U., Joshi, N.V. and Gadgil, M., 1998. On the patterns of tree diversity in the Western Ghats of India. Current science. 75: 594-602. Gilliam, F.S. 2002. Effects of harvesting on herbaceous layers diversity of a central Appalachian hard wood forest in west Virginia, USA. Forest Ecology and Management 155: 33-43. Hara, M., Hirata, K., Fujihaxa, M., Oona, K. and Hsich, C.F., 1997. Floristic composition and stand structure of three evergreen broad-leaved forests in Taiwan, with special reference to the relationship between Micro-landform and vegetation pattern. National Historical Research (Special Issue) 4: 81-112. Hubbell, S.P., 1979. Tree dispersion, abundance and diversity in a tropical dry forest. Science. 203: 1299-1309. Jeffre, T. and Veillon, J.M. 1990. Etude floristique et structurale de deux forets denses humides surroches ultrabasiques en Nouvelle-Caledonie. Bull. Mus. Hist. Nat. Paris. 12(B): 243-73. Kadavul, K., and Parathasarathy, N., 1999b. Structure and composition of woody species in tropical semi-evergreen forest of Kalrayan hills, Eastern Ghats, India. Tropical Ecology. 40: 77-90. Kadavul, K., and Parthasarathy, N., 1998. Biodiversity of woody species and conservation of tropical semi-evergreen forest in Kalrayan hills, Eastern Ghats, India. Trop. Ecol. (submitted). Kadavul.K., and Parthsarathy.N., (1999a) plant biodiversity and conservation of tropical Semi-evergreen forest in the Shervarayan hills of Eastern ghats, India. Bio-diversity and conservation. 8 : 421 439. Khera, N., A. Kumar, J. Ram & A. Tewari. 2001. Plant biodiversity assessment in relation to disturbances in mid elevational forest of Central Himalaya, India. Tropical Ecology 42: 83-95. Koh, L.P., R.R. Dunn, N.S. Sodhi, R.K. Colwell, K.C. Proctor & V.S. Smith. 2004. Species coextinctions and biodiversity crisis. Science 305: 1632-1634. Lakshmi, G., 1995. Ecological studies on the vegetation of Kolli hills, Salem (Dt.) Tamil Nadu, Ph.D thesis, Bharathiar University, Coimbatore. Lieberman, D., Lieberman, M., Hartshorn, G.S. and Peralta, R. 1985. Growth rates and age-size relationships of tropical wet forest trees in Costa Rica. Journal of Tropical Ecology. 1: 97-109. Liebernman, D., Lieberman, M., Peralta, R and Hartshorn, G.S. 1996. Tropical forest structure and composition on large-scale altitudinal gradient in Costa Rica. Journal of Ecology. 84: 137-152. Margalef, R., 1968. Perspectives in Ecological Theory. Univ. of Chicago Press. pp. 111. Menhinick, E.F., 1964. A comparision of some species diversity indices applied to samples of field insects. Ecology. 45: 859-861. MoEF. 1999. National Policy and Macrolevel Action Strategy on Biodiversity. Ministry of Environment and Forest, Government of India, New Delhi. Myers, N., 1984. The Primary source: Tropical forests and our future. Norton, W.W. Norton, New York. Nadkarni, N.M.,, Matelson, T.J., and Haber, W.A., 1995. Structural characteristics and floristic composition of a neotropical cloud forest, Monteverde, Costa Rica. Journal of Tropical Ecology. 11: 481-495. Odum, E.P., 1971. Fundamentals of Ecology. 3rd edn. W.B. Saunders Co., Philadelphia. Ojo Lo and Ola-Adams, B.A., 1996. Measurement of tree diversity in the Nigerian rainforest. Biodiversity and Conservation. 5: 1253-1270. Paijmans, K., 1970. An analysis of four tropical rain forest sites in New Guinea. Journal of Ecology. 58: 77-101. Parathasarathy, N. and Karthikeyan, R., 1997a. Biodiversity and population density of woody species in ,a tropical evergreen forest in Courtallum reserve forest, Western Ghats, India. Trop. Ecol. 38:297-306. Parthasarathy, N., and Karthikeyan, R., (1997a) Plant biodiversity inventory and conservation of two tropical dry evergreen forests or the coromandal coast, South India. Biodiv. Conserv.6, 1063 83. Parthasarathy, N., 1999. Tree diversity and distribution in undisturbed and human impacted sites of tropical wet evergreen forest in Southern western ghats, India. Biodiversity and conservation. 8: 1365-1381. Parthasarathy, N., and Karthikeyan, R., 1997b. Plant biodiversity inventory and conservation of two tropical dry evergreen forest on the Coromandel coast, South India. Biodiversity and conservation. 6: 1063-1083. Parthasarathy, N., and Pia Sethi, P., 1997. Trees and liana species diversity and population structure in a tropical dry evergreen forest in South India. Tropical Ecology. 38: 19-30. Pascal, J.P., and Pelissier, R., 1996. Structure and floristic composition of a tropical evergreen forest in south west India. Journal of Tropical Ecology. 12: 191-214. Pianka, E.R., 1966. Latitudinal gradients in species diversity: a review of concepts. American Naturalist. 100: 33-46. Pickett, S.T.A. & P.S. White. 1985. The Ecology of Natural Disturbance and Patch Dyanamics. Acadimic Press, Orlando, Florida. Pielou, E.C., 1966. The measurement of diversity in different types of biological collections. J. Theor. Biol. 13: 131-144. Pimm, S.L. & P. Raven. 2000. Extinction by numbers. Nature 403: 843-844. Poore, M.E.D., 1968. Studies in Malaysian rainforest. 1. The forest on Triassic sediments in Jengka forest reserve. Journal of Ecology. 56: 143-196. Puyravaud, J.P., D. Shridhar, A. Gaulier, S. Aravajy & S. Ramalingam. 1995. Impacts of fire on a dry deciduous forest in the Bandipur National Park. southern India: preliminary assessment and simplification for management. Current Science 68: 745-748. Puyravaud, J.P., Sridhar, D., Gaulier, A., Aravajy, S., and Ramalingam, R., 1995. Impact of fire on a dry deciduous forest in Bandipur National Park, Southern India. Preliminary assessment and implications for management. Current Science. 68: 745-751. Rajan, P., S.M. Sundarapandian, S. Chandarasekaran & P.S. Swamy. 1995. Variations in soil seed bank at different microsites in a deciduous forest near Madurai, south India. International Journal of Ecology and Environmental Sciences 21: 263-272. Reese, H., Nilsson,M., Granqvist Pahlen, T., Hagner, O., Joyee S., Tingelof, U., (2003). Country wide estimates of forest variables using satellite data and field data from the National Forest Inventory. Ambio, 32, 542-548. Schumann, M.E., A.S. White & J.W. Witham. 2003. The effects of harvest created gaps on plant species diversity, composition and abundance in a Maine Oak-pine forest. Forest Ecology and Management 176: 543-561. Simpson, E.H.,1949. Measurement of Diversity. Nature (London). 163: 688. Singh A., Reddy V.S., and Singh J.S., 1995. Analysis of woody Vegetation of Corbett National Park, India Vegetation 120: 69 79. Sousa, W.P. 1984. The role of disturbances in natural communities. Annual Review of Ecology and Systematics 15: 353-391. Sukumar, R. Dattaraja, H.S., Suresh, H.S., Radhakrishnan, J., Vasudeva, R., Nirmala, S., and Joshi, N.V., 1992. Long-term monitoring of vegetation in a tropical deciduous forest in Mudumalai, Southern India. Curr. Sci. 62: 608-616. Swamy P.S., Sundarapandian S.M., Chandrasekar P. and Chandrasekaran S.2000. Plant species diversity and population structure of a humid tropical forest in Tamilnadu, India. Biodiversity and conservation. 9: 1643 1669. Thorington, R.W.Jr, Tannenbaum , B., Tarak, A. and Rundran,, R.1982. Distribution of trees on Barro Colorado Island: a five hectare Sample. In: Leigh E.G. Jr. Rand, A.S. and Windsor, D.M. (eds). The Ecology of a Tropical Forest. seasonal Rhythms and Long-term changes Smith sonian Institution Press, Washington, D.C. UNEP, 2001. India: State of the Environment-2001. United Nations Environment Programme. Nairobi. Venkateswaran, R., and Parthasarathy, N., 2003. Tropical dry evergreen forests on the Coromandel Coast of India: structure, composition and Human disturbance. Ecotropica. 9: 45-58. Whitmore, T.C., 1984. Tropical rain forest of the Far Fast. 2nd edn. Oxford University Press, Oxford, U.K. 352 pp.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||