Assessment of variation in some medicinal plant species envisaged of having the potential for the preservation of herbal products using some statistical models
Y. Ameyaw*1, Duker-Eshun2, F. C. Mills-Robertson3
1. Plant Development Department, 2. Phytochemistry Department and
3. Microbiology Department, Centre for Scientific Research into Plant
Medicine, Mampong- Akuapem, E/R.
** For all correspondence: [email protected]
Abstract
A survey research was conducted on some medicinal plant species envisaged of having the potential for preservation of herbal preparations. The aim was to determine whether there is an interspecific relationship among some selected medicinal plant species using their total extracts as the basis for computerization. To this end, statistical model comprising the Duncan’s Multiple Range Test and Principal Component Analysis (PCA) were applied to the total extract obtained from the medicinal plant species harvested from Mampong - and Mamfe – Akuapem environs to determine the existence of variations. The results showed the existence of variation and some of the medicinal plant species were more influential or weighted more than others.
Keywords: Anti-microbial, preservative, principal component analysis.
Introduction
For centuries, plant-based medicaments have been man’s prime therapeutic weapons and are still in the frontline for treating a large number of diseases. Furthermore, the deeper one goes into the study of plants used in traditional medicine, the more one perceives new possibilities for additional plant derivatives. Man’s survival has been dependent on his inmate curiosity, his desire to examine by trial and error all aspects of his environs and conclude on which ones are harmful and which ones give him the greatest nourishment.
According to Saxena et al. (1994 and 1995), screenings of several medicinal plants in various laboratories have proved to have antimicrobial properties. The petroleum ether extract of the fruits of Xylopia aethiopica (Dunal) A. Rich is known to have antifungal properties as well as a wide-range of antibacterial activities as a results of the presence of xylopic acid (Abegaz et al., 1977; Boakye-Yiadom et al., 1977). Pruthi et al., (1980) also noted the prevention aspects of the ethanolic extracts of Cinnamomnm zeylanium on the growth of moulds, yeasts and bacteria. Cinnamomnm zeylanium and Zanthoxylum xanthoxyloides have been found to exhibit antimicrobial properties (Anon. 1992). The aerial parts of the medicinal plant part of Hoslundia opposita is known to treat gonorrhooea, cystitis, hookworm, cough, fevers, colds, wounds and bilharzias (Chhabra and Uiso, 1994; Ngadjui et al., 1991; Hedberg et al., 1983). The alkaloidal extracts of Annona crassiflora Linn.have been reported to exhibit analgesic, spasmolytic and limited anti-bacterial effects (Hocquemiller et al., 1982). Extraction of the whole plant of Cnestis ferruginea is used to treat conjunctivitis, syphilis, gum pain, wounds, diarrhoea and dysentery (Bouquet and Debray, 1974; Le Grand, 1989). As stated in Ghana Pharmacopoiea (1992), Psidium guajava is used to manage acute and chronic diarrhoea in addition to Herpes zoster.
From the above plethora of medicinal plant species, which are used locally in some communities in Ghana for the preservation of decoctions, concoction and poultice, it could be envisaged that they could be used for the preservation of herbal products, in place of chloroform, which has carcinogenic effects. This has prompted researchers at the Centre for Scientific Research into Plant Medicine to screen a host of medicinal plants having the potential in promoting the shelf life of herbal products or preparations by assessing the level of variation using the weighting of the plant extracts.
According to Whang, et al., (2002), variation patterns at the species level and below are based on multivariate statistical methods. As observed by Sneath and Sokal (1973) multivariate analysis has proven useful in systematics, particularly at the species level and below, since it helps to approximate the scientific goals of objectivity, repeatability, flexibility and quantitatively.
Therefore, the research is aimed at establishing the interspecific variations among the plant species, using their total extracts through the application of the Duncan’s Multiple Range Test and Principal Component Analysis.
Materials and methods
Collection site
The medicinal plants listed below were collected from the environs of Mampong-Akuapem and Mamfe-Akuapem in the Akuapem North, Esatern Region as well as Ayikuma in the Greater-Accra Region.
List of medicinal plant species
Plant species Plant part used Coding
Alchornea cordifolia (Schum.& Thonn.) Stapf Leaves ACl
Cinnamomum zeylanium Nees Leaves CVl
Clausena anisata (Will.) Hook.f.ex Benth Root bark CArb
Cryptolepis sanguinolenta (Lindl.) Schtr. Root bark CSrb
Cnestis ferruginea DC Leaves CFl
Hoslundia opposita Vahl Leaves HOl
Lippia multifolia Moldenke Leaves LMl
Morinda lucida Benth Leaves MLl
Ocimum gratissimum Linn. Leaves OGl
Psidium guajava Linn. Leaves PGl
Spondias mombin Linn. Leaves SMl
Tridax procumbens Whole plant TPwp
Xylopia aethiopica (Dunal) A. Rich Fruits XAf
Zanthoxylum xanthoxyloides (Lam.) Waterm Root bark ZXrb
Authenticity of the medicinal plants
Seasoned Botanists did the authentication of the medicinal plant species in the Herbarium of the Centre for Scientific Research into Plant Medicine. Voucher specimen of the plant species have been deposited in the Centre’s Herbarium.
Experimental
Quantitative determination of total extracts
Quantitative determination of total extracts was carried out, five replicates were prepared for each plant organ per location and the mean value computed. The plant materials were air-dried for 30 days and ground to fine powder using the Manesty disintegrator. Equal quantities of 1000g of the powders obtained in each case were soxhlet extracted with hexane for 12 hours to defat the powdered plant materials. The defatted powder in each case was taken and the alkaloid extracted with ethanol. The extracts were filtered and concentrated under reduced pressure using a rotary evaporator. The residue was mixed with 200ml of 10% aqueous acetic acid and allowed to stand overnight. The mixture was filtered with Whatman No. 1 filter paper. The filtrate was basified to pH 10 with ammonium solution. The basic mixture was extracted with two equal volumes of 200ml of chloroform. The chloroform extract was dried with anhydrous sodium sulphate and the solvent removed under reduced pressure.
Statistical analysis
The Minitab 13.32 was fitted to the dataset to determine whether there is variation among the medicinal plant species obtained from the sample locations. The Duncan’s Multiple Range Test and Principal Component Analysis were applied to assess the level of significant differences and weighting of the medicinal plant species, respectively.
Results
The results presented in Tables 1 and 2 below, show the significant difference and weightings among the medicinal plant species using Duncan’s Multiple Range Test and the Principal Coponent Analysis (PCA).
Table 1: Showing levels of variation among the medicinal plant species using the Duncan’s Multiple Range Test.
|
Name of plant species |
Family |
Local name |
Plant part used |
Total extraction (g) |
|
Alchornea cordifolia |
Euphorbiaceae |
Agyamma, Gyeka, Gboo, Gbloo, Ahama |
Leaves |
28.30(g) |
|
Cinnamomum zeylanium |
Lauraceae |
--------- |
Leaves |
83.10 (b) |
|
Clausena anisata |
Rutaceae |
Sesadua, SamanƆbere, Duawonsi, Ayira |
Leaves |
47.59 (c) |
|
Cryptolepis sanguinolenta |
Periploaceae |
Nibima, Kadze, Gangamau |
Root bark |
19.68 ( j ) |
|
Cnestis ferruginea |
Connaraceae |
ApƆsẽ, Akitase, Pudaegye |
Leaves |
18.43 (k) |
|
Hoslundia opposita |
Labiate |
Aberewa-ani-nsu, Nunum-nini, Asifuaka, AkƆta |
Leaves |
32.65 (f) |
|
Lippia multifolia |
Verbenaceae |
Saanunum, Na suru, Afu-loti |
Leaves |
23.28 (i) |
|
Monodora myristica |
Annonaceae |
Awerewa, wereƐ., Abotokuradua, Avonoba, Maalai, Yikwi |
Seeds |
18.06 (k) |
|
Morinda lucida |
Rubiaceae |
KƆnkroma |
Leaves |
32.43 (f) |
|
Ocimum gratissimum |
Labiate |
Onumum, Suru, Sru,GbekonƆ |
Leaves |
39.70 (e) |
|
Psidium guajava |
Myrtaceae |
Oguawa, Eguaba, Gowa, Goa, AduƆba |
Root bark |
99.66 (a) |
|
Spondias mombin |
Anacardiaceae |
Atoaa, atawa, adontsho, toroma, adombaatsho |
Leaves |
6.16 (l) |
|
Tridax procumbens |
|
|
Whole plant |
24.16 (h) |
|
Xylopia aethiopica |
Annonaceae |
Hewentia, Soo, Tsuo |
Fruit |
47.40 (cd) |
|
Zanthoxylum xanthoxyloides |
Rutaceae |
ƆKăntő, Yea, Bɛbun, Kanfu, HaatƩo |
Root bark |
47.10 (d) |
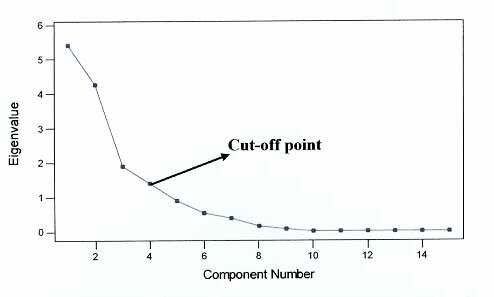
Principal Component Analysis was applied to the mean values of the plant extract. The 4 components that had eigenvalues that summed to > 1 were extracted (Table 2 below); therefore, the cut-off point was pegged on the 4th PC or eigenvalue. The results of the Scree plot in Fig. 1 show that fifteen (15) principal components were extracted or recorded; four Principal Components were considered acceptable although the elbow/change in slope is on the 10th Principal Component. This is because the total cumulation of the extracted components could accommodate 86.4% of variation in the original data (Fig. 1).
Fig. 1: A Scree plot showing the relationship between computational
eigenvalues and component numbers based on the total extract of the medicinal plant species.
Table 2. Principal Component Analysis (PCA) rotated using varimax method based
on the total extracts of the medicinal plant species converged in 15
iterations.
Plant species |
PC 1 |
PC 2 |
PC 3 |
PC 4 |
|
ACl |
0.080 |
0.383 |
-0.094 |
0.390 |
|
CZl |
0.346 |
-0.016 |
-0.147 |
-0.093 |
|
CArb |
-0.162 |
-0.436 |
-0.054 |
0.064 |
|
CSrb |
-0.405 |
0.070 |
-0.120 |
-0.139 |
|
CFl |
0.167 |
-0.339 |
0.092 |
-0.310 |
|
HOl |
0.361 |
0.025 |
-0.024 |
-0.201 |
|
LMl |
-0.047 |
0.175 |
-0.558 |
0.059 |
|
MMs |
0.158 |
0.078 |
-0.610 |
0.070 |
|
MLl |
0.169 |
-0.201 |
-0.437 |
-0.381 |
|
OGl |
-0.405 |
0.070 |
-0.120 |
-0.139 |
|
PGrb |
-0.405 |
0.070 |
-0.120 |
-0.139 |
|
SMl |
-0.317 |
-0.040 |
-0.089 |
0.132 |
|
TPwp |
0.135 |
-0.239 |
-0.068 |
0.649 |
|
XAf |
-0.065 |
-0.450 |
-0.127 |
0.209 |
|
ZXrb |
-0.131 |
-0.441 |
-0.110 |
0.072 |
|
Eigenvalue |
5.4199 |
4.2637 |
1.8981 |
1.3845 |
|
Proportion |
0.361 |
0.284 |
0.127 |
0.092 |
|
Cumulative |
0.361 |
0.646 |
0.772 |
0.864 |
![]() In Table 2 above, the cumulated percentage of variance under the Principal Component One (1) is 36.1. It implies that 36.1% of the primary variables have been extracted leaving a further 63.9%. This calls for further extraction of the variables leading finally to 86.4% of cumulated % of variances under the PC 4, which is expressed in the Table 2. In distinguishing high component, weights with values of 0.4 as their absolute value were arbitrarily adopted as the biological standard. From the above Table, the component or weights of Cryptolepis sanguinolenta (CSrb at - 0.405), Ocimum gratissimum (OGL at
In Table 2 above, the cumulated percentage of variance under the Principal Component One (1) is 36.1. It implies that 36.1% of the primary variables have been extracted leaving a further 63.9%. This calls for further extraction of the variables leading finally to 86.4% of cumulated % of variances under the PC 4, which is expressed in the Table 2. In distinguishing high component, weights with values of 0.4 as their absolute value were arbitrarily adopted as the biological standard. From the above Table, the component or weights of Cryptolepis sanguinolenta (CSrb at - 0.405), Ocimum gratissimum (OGL at
- 0.405) and Psidium guajava (PGrb at – 0.405) were the most influential variables in the first (1) principal component (PC1). In the second principal component (PC2), the component or weights of Cinnamomum zeylanium (CZrb at - 0.436), Xylopia aethiopica (Xaf at – 0.450) and Zanthoxylum xanthoxyloides (ZXrb at - 0.441) were also the most influential variables. In the third PC, Lippia multifolia (MLl at – 0.558) and Monodora myristica (MMs at – 0.610) were noted to be the influential plant species. Finally, Tridax procumbens (TPwp at 0.649) was the only influential plant species among the lot.
Discussion
The results in Table 1 prove that the plant extracts of the medicinal plant species were almost statistically variable. Results presented in Figs. 1 and Table 2 on the plant extracts indicate that some of the medicinal plant species can be rated as highly variable or weighted more than others. These led to change in slope or elbow of the Scree plots or the extraction of certain plant extracts more than others as expressed by the various Principal Component or factor loadings (PCs) above, which might have resulted from soil differences and environmental fluctuations as the main causative agents. Similar reports on the correlation weighting or discriminant of characters, have earlier been expressed by Whang, et al., (2002), Sneath and Sokal (1973).
Conclusion
Some of the characters were more influential or weighted more than others, although, all the characters were statistically, treated equally.
Acknowledgement
The authors would like to thank the Council of the Centre for Scientific Research into Plant Medicine, Mampong – Akuapem, E/R – Ghana.
References
Anon. (1992). “Ghana Herbal Pharmacopoeia”, Policy Research and Strategic Planning Institute, pp. 152.
Berhanu Abegaz and Paulos G. Yohannes (1977). Phytochemistry, 21, 1791.
Boakye-Yiadom, K., Fiagbe, N. I. Y. and Ayim, J. S. K. (1977). Lloydia, 40, 453.
Bouquet, A. and Debray, M. (1974). Medicinal plants of Ivory Coast. Trav. Orstom. 32, 1.
Chhabra, S. C. and Uiso, F. C. (1994). Antibacterial activity of some Tanzanian plants used in traditional medicine. Fitoterapia. 62(6): 499 – 503.
Technology Transfer Centre (TTC) (1992). Ghana Herbal Pharmacopoiea. Advert Press, Accra.
Hedberg, I, Hedberg, O., Madati, P. J., Mshigeni, K. E., Mshiu, E. N., Samuelson, G. (1983). Inventory of plants used in traditional medicine in Tanzania. II. Plants of the families Dilleniaceae – Opiliaceae. J. Ethnopharmacol. 9 (1): 105 – 127.
Hocquemiller, R., Rasamizafy, S. and Cave, A. (1982). Tetrahedron. New York, U. S. A. 38: 911.
Le Grand, A. (1989). Antiinfective phytotherapy of the tree-savannah, Senegal (Western Africa) III: A review of the phytochemical substances and anti-microbial activity of 43 species. J. Ethnopharmacol. 25 (3): 315 – 338.
Ngadjui, B. T., Ayafor, J. F., Sondengam, B. L., Connolly, J. D., Rycroft, D. S. (1991). Hoslundin, Hoslundial and Hoslunddoil: Three new flavonoids from the Hoslundia opposita (Lamiaceae). Tetrahedron 47 (22): 3555 – 3564.
Pruthi, J. S. (1980). Spices and Condiments Chemistry. Advances in Food Research-Supplement 4. New York Academic Press, pp. 24.
Saxena, G., McCutucheon, A. R., Farmer, S., Towers, G. H. N. and Hancock, R. E. W. (1994). Antimicrobial constituents from Rhus glabra. Bong Int. J. Pharmacog. 33: 33 – 36.
Saxena, G., Farmer, S. and Towers, G. H. N. (1995). Antimicrobial compounds from Alnus rubra. J. Ethnopharmacol., 5: 196 – 199.
Sneath and Sokal (1973). Numerical taxonomy: The principles and practice of numerical classification. Freeman and company, San Francisco.
Whang, S. S., Choi, K., Hill, R. S. and Jaehong Pak, 2002. A morphometric analysis of infraspecific taxa within the Ixeris chinensis complex (Asteraceae, Lactuceae); Bot. Bull. Acad. Sin. 43: 131-138.