|
Ethnobotanical Leaflets 12: 542-52. 2008.
Intra-Specific Genetic Relationship Analyses of Cinnamomum trivancoricum Based on GC-MS Volatile Oil Markers
Muthiah Maridass
Animal Health Research Unit, St. Xavier’s College (Autonomous), Palayamkottai-627002, Tamil Nadu, India Email: [email protected]
Issued 25 July 2008
Abstract Cinnamomum trivancoricum (Lauraceae) is an endemic species to India. The identification and genetic relationship of these species were studied based mainly on volatile oil constituents and their retention time. The color of essential oils was brown and the number of components of oils that were identified from Kodaiyar, Karaiyar, Thenmalai and Kodaikkanal samples were 22, 20, 19 and 18 which accounts 85.6%, 85.4%, 91.26% and 85% of their contents, respectively. Among them, terpinen-4-ol, trans- cinnamaldehyde, a-terpineol, and β-caryophyllene were the major compounds. A Dendrogram based on Jaccard’s similarity coefficients indicated that the distribution pattern of the four accessions was coherent with their geographical origins. Most of the genetic variation (volatile oils Retention time data) occurred among clones within each region. However, the very close relationship between Kodaiyar and Thenmalai populations are statistically significant (P<0.001). This is the first report regarding the inheritance of volatile oils elution time and their application on genetic diversity of wild populations, and it provides useful baseline data for optimizing sampling strategies in breeding. These results are important for future genetic improvement, identification, and conservation of Cinnamomum trivancoricum germplasm. Key Words: Cinnamomum trivancoricum; Barks; Volatile oils; Chemical diversity; genetic diversity. Introduction Plant genetic resources for food and agriculture are the basis of global food security. They comprise diversity of genetic material contained in traditional varieties, modern cultivars, crop wild relatives and other wild species. The main aim of genetic resource conservation is to conserve as wide a representation as possible of the array of extent genetic variations of target taxa (Ferguson et al., 1998). This is irrespective of the relative frequency of any gene or linked gene complex in germplasm. Satisfying this objective is dependent in part on the efficiency of selection of species and location for the sampling of the genetic diversity. Most species display a complex of genetic variations along their range of distribution (McCall et al., 2004, Miller and Schaal,2006). For landraces, this is a function of species characteristics, such as breeding system, migration and dispersal mechanisms, which determine the movement of genes among populations (Erskine, 1997, Herlihy and Eckert, 2004) biotic pressure, for example, competition, predation and local anthropogenic influence and biotic selection intensities determined by location (Ferguson et al.,1998). Genetic conservation strategies are initially concerned with understanding of the genetic variation within species and then by the geographical distribution of genetic variation (Frankel et al., 1995; Ferguson et al., 1998). Cinnamomum trivancoricum Gamble (Family: Lauraceae) is an endemic plant, which is widely distributed in the higher elevation of Southern Western Ghats, South India and leaves and barks are used as additive in foods to offer aroma and flavor. Molecular markers have been applied to study of genetic diversity from natural populations and formulate efficient sampling strategies to capture maximum variation for genetic resources conservation. Among several characters such as phenolic compounds, flavonoids, glycosides and terpenoids have been used more commonly. Therefore, in the present study performed to GC-MS analysis of volatile oils constituents of four accessions of C. trivancoricum barks were collected in South India. Materials and Methods
Plant material The plant material (barks) of 4 accessions was collected during the flowering period (May-August, 2001) from the field collection of the Southern Western Ghats, Tamil Nadu, India. Voucher specimens of each field accession were deposited in the Animal Health Research Unit, St. Xavier’s College (Autonomous), Palayamkottai, Tamil Nadu, India. Essential oils analysis
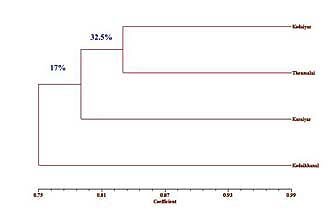
One hundred gm of fresh barks were subjected to hydrodistillation for 3hr at 100oC in a Clevenger apparatus for extraction of the essential oil. The essential oil was subsequently cooled, separated from the condensation water and analyzed by the GC-MS. Sample (0.1µl) of oil diluted with pentane (1:10,000,v:v) were analysed on an HP-GCD apparatus equipped with an HP5 (30m x 0.25mm) fused-silica capillary column using helium(1ml/min) as a carrier gas. The injector and detector temperatures were 250 and 280 oC, respectively, and the oven conditions were 70 oC for 2 min, then rising from 70 to 200 oC at a rate of 4 oC/min and subsequently held at 200 oC for 10 min. The mass range was recorded from 45 to 450 m/z, with ionization energy of 70 eV. Major components were identified by co-injection with authentic standards and by with recorded from computerized libraries. Quantification of compounds was based on a comparison of the total ion chromatographic peaks size with those obtained with internal standard. The constituents of the oil were identified by the combination of mass spectral and retention indexes and they were compared with both those of reference authentic compounds and from library spectra data and literature (Adams, 1995; Jennings and Shibamoto, 1980). Data analysis The chromatogram peaks were converted into a “1” and “0” matrix, to indicate the presence or absence of a peak, respectively. Genetic similarities (GS) were estimated for all comparisons of each accessions samples according to Nei (1972) as GS=2nxy/(nx+ny) in which nx and ny are the total numbers of peaks in the chromatograms of the samples x and y, respectively, and nxy is the number of peaks shared by the two samples. To examine the genetic relationships between populations, a dendrogram was constructed by an unweighted paired group method of cluster analysis using arithmetic averages (UPGMA) option of the SPSS (version 11.0) software. After this process, GC-MS chromatograms of all C. trivancoricum barks were made and then data with different peak retention times from each accession were compared. Results and Discussion A comparison is shown in Table -1 of volatile oil of C. trivancoricum bark distilled from the different accessions (Kodaiyar, Karaiyar, Thenmalai and Kodaikkanal). The hydrodistillation of the barks yielded (%) brown oil with an aromatic odour (Table-1). The essential oils from five natural populations of C. trivancoricum in South India were analyzed for quality and quantity using GC-MS (Table- 2). Twenty-seven essential oil compounds were obtained, in which twenty five were identified. The identified compounds represented in Table-2. Among them, terpinen-4-ol, trans- cinnamaldehyde, a-terpineol, and β-caryophyllene were the major compounds. Among the four accessions, the maximum percentages of terpinen-4-ol over the total compounds 25.6%, 23.5%, 24.9%, and 26.1%, respectively (Table-1). GC/MS analysis resulted in detection and identification of volatile constituents of four accessions of different locality (Table-2). Volatile constituents showed high variability among four accessions of C. trivancoricum (Table-3). On the based on retention time of 27 constituents were cluster analysis of four accessions represented in (Fig.1), thus indicating close resemblance in the chemical content of C. trivancoricum around the Kodaiyar and Thenmalai. The genetic distances, Jaccard’s coefficient of similarity, among C. trivancoricum accessions were based on retention time of elution of volatile compounds (Tab-2). Jaccard’s similarity coefficients ranged from 0.83 (between an accession from Thenmalai and accessions from the Kodaiyar, and Karaiyar) to 0.76 (between the accession from Karaiyar and accessions from the Kodaikkanal), with an average of 0.65%. The dendrogram produced using distance matrix on average linkage (Fig. 4) shows three groups of germplasm; one group consisted of very closely related germplasm from Kodaiyar and Thenmalai region, except the accession from Karaiyar and Kodaikkanal. However, the degree of this variability is different with the first time using tools of volatile oils (Retention time). The Mantel test, used to compare essential oil content to the genetic matrices, indicated a low, did not significant relation between the matrices, suggesting that there was no chemotypic differentiation. The genetic differentiation of accessions of C. trivancoricum could be broadly explained as a result of abiotic (geographical, e.g., hydrographic connections, or climatic differentiation. e.g., annual rainfall differences) and biotic (pollination between populations and seed dispersal) factors. The percentage of polymorphism i.e., 49.61 was higher in comparison to other endangered plants, e.g. Lactoris fernandeziana (Lactoridaceae) (24.5%) (Brauner et al., 1992), Paeonia suffruticosa (22.5%) and Paeonia rockii (27.6%) (Pei et al., 1995), Cathaya argyrophylla (32%) (Wang et al., 1996), and Dacydium pierrei (33.3%) (Su et al., 1999). This shows that the species genetic diversity by itself is low, but relatively higher when compared to other endangered species as stated above and it should be able to adapt to the environmental variation. The present results represented the volatile oils content variability of different accessions of different altitude populations of C. trivancoricum, which exposed a high variation in the chemical composition and quantity of essential oil. These results concluded that differences in ecological factors (growth season and other environmental variables) and genetic differences among chemotypes most likely affected the essential oil constituents measured in this study. Conclusion As a result of this comparative investigation of different accessions of essential oils of C. trivancoricum in Southern India, it was found that the composition of these oils differed significantly only in quantity and quality. For the first time, my preliminary genetic chemical diversity study of volatile oils constituents of Cinnamomum trivancoricum using GC-MS analysis volatile markers. Further study should be going on primary metabolites using genetic diversity of Cinnamomum species of India. Acknowledgements The author thanks to SERC-Division, Department of Science and Technology, Govt. of India, New Delhi for financial support. References Adams, R. P. 1995. Identification of essential oils components by gas chromatography/mass spectroscopy. Allured Publ. Corp., Illinois. Jennings, W. and Shibamoto, T. 1980. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography. Academic Press, New York. Ferguson, M. E., Robertson, L. D., Fordlioyd, B. V., Newbury, H. J. and Maxted, N., 1998: Contrasting genetic variation amongst lentil landraces from different geographic origins. Euphytica, 102: 265–273. Mccall, A. C., Kelly, D. and Chapman, H. M.,2004. Little geographic or host plant genetic variation in a Chionochloa. (Poaceae) seed predator (Cecidomyiidae: undescribed species). New Zeal. J. Ecol., 28: 215–224. Miller, A. J. and Schaal, B. A., 2006: Domestication and the distribution of genetic variation in wild and cultivated populations of the Mesoamerican fruit tree (Spondias purpurea L. Anacardiaceae). Mol. Ecol., 15: 1467–1471. Erskine, W.(1997). Lessons for breeders from landraces of lentil. Euphytica, 93: 107–112. Herlihy, C. R. and Eckert, C. G. (2004). Experimental dissection of inbreeding and its adaptive significance in a flowering plant, (Aquilegia canadensis Ranunculaceae). Int. J. Org. Evolution, 58: 2693-703. Frankel, O., Brown, A. H.D. and Burdon, J.J. (1995). The conservation of plant biodiversity. Cambridge University Press, Cambridge. Wang, X.Q., Zou, Y.P., Zhang, D.M., Hong, D.Y., (1996). RAPD analysis for genetic polymorphism in Cathaya argyrophylla. Sci. China., 26: 437- 441. Su, Y.J., Wang, T. and Huang, C. (1999). RAPD analysis of different population of Dacydium pierrei. Acta Bot. Sin., 40:169-175. Pei, Y.L., Zou, Y.P., Yin, Z., Wang, X.Q., Zhang, Z.X. and Hong, D.Y. (1995). Preliminary report of RAPD analysis in Paeonia suffruticosa subsp. Spontanea and P. rockii. Acta Phy. Sin., 33: 350-356.
Fig. a and b. Cinnamomum trivancoricum barks and fruits.
Table 1: Comparison of oil yields (oven dry weight basis) for different accessions of C. trivancoricum
Table-2: Volatile oils composition (%) of different accessions of C. trivancoricum barks
Table-3: Converted data on elution of active constituent’s retention time of essential oils peaks in different accessions of C. trivancoricum barks
Table- 4: Percentage of similarities between four accessions of C. trivancoricum basing on volatile oils retention time
Fig.1: Dendrogram showing intra genetic relationships of C. trivancoricum based on retention time of volatile oils constituents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||