|
Ethnobotanical Leaflets 12: 1145-52. 2008.
Phytochemical Investigations of some Laticiferous Plants belonging to Khandesh Region of Maharashtra
Mahajan R. T.1 and Badgujar S. B.2*
1Postgraduate Department of Biotechnology, Moolji Jaitha College, Jalgaon, Maharashtra 2 Department of Biotechnology, SSBT’s, College of Engineering and Technology, Bambhori, Post Box No. 94, Jalgaon: 425001, Maharashtra, India *Correspondent author: E – Mail: [email protected]
Issued 01 December 2008
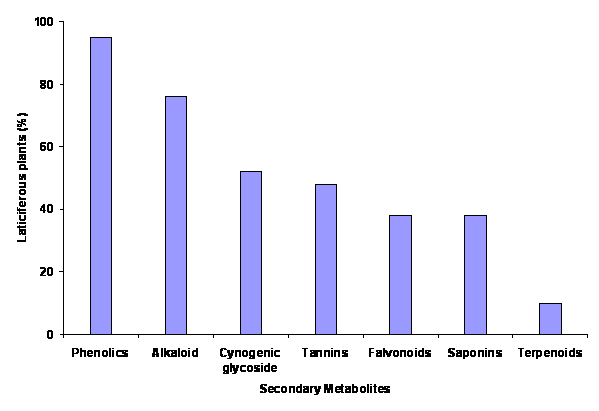
ABSTRACT Analyses were carried out on latex obtained from twenty one plant species belonging to Khandesh region of Maharashtra, India, for the presence of possible secondary metabolites, namely alkaloids, flavonoids, terpenoids, cynogenic glycosides, phenolics, tannins and saponins. Phenolic compounds were found in all latex samples except in Ipomoea carnea Jacq. Seventy six percent species of laticiferous plants contain alkaloid. Terpenoids were found in the latex of Carica papaya L. and Manilkara zapota (L.) P. van Royen only however, remaining plants were devoid. Cynogenic glycosides and tannins were detected in 52 % and 48 % of latex samples respectively. Flavonoids and saponins were detected in equal number of latex samples i.e. 38 %. Out of this, four species of Apocynaceae and single species of Euphorbiaceae, Moraceae, Carricaceae, Convolvulaceae etc. showed flavonoid. While three species of Euphorbiaceae, two species of Apocynaceae and Moraceae and single species of Asclepiadaceae showed saponin. The moisture and total solid content varies from species to species in latex samples analyzed. Euphorbia prunifolia Jacq. had highest level of moisture i.e. 93.33% and less solid content i.e. 6.67% whereas Euphorbia hirta L. had lowest level of moisture i.e.63.63% and highest content of total solid i.e. 36.37%. The results suggest that, the laticiferous plants would be exploited in the management of various diseases as they have diverse group of secondary metabolites. Keywords: Laticiferous plants, Latex, Secondary metabolites, Phytochemical analysis.
INTRODUCTION Accumulated evidences indicate that the latex bearing plants used in the management to cure various diseases such as diabetes, asthma, dysentery, diarrhea, malaria and skin problems (Nadkarni, 1976) and allied industrial applications also (Wealth of India, 1948). Plant latex is the milky juice, found in long branching tubes known as latex tubes. This juice is white, yellow or pinkish in colour. It is a viscous fluid and colloidal in nature. Known ingredients of latex are proteins, alkaloids, tannins, terpens, starch, sugars, oils, resins, gums and enzymes (Pandey, 2001). Ipomoea carnea Jacq. and Euphorbia hirta L. are reported for wound healing activity and flavonoids (Ambiga et al, 2007 and Jaiprakash et al, 2006). Latex of Calotropis procera (Ait.) R.Br. was described for wormicidal activity (Shivkar and Kumar, 2003) and larvicidal activity (Badgujar and Mahajan, 2008). Curcain a proteolytic enzyme isolated from latex of Jathropha curcas Linn has been reported for wound healing activity (Nath and Dutta, 1992). Alstonia scholaris R. Br. is well-known for various activities viz., antimicrobial, antiamoebic, antidiarrhoeal, antiplasmodial, hepatoprotective, immunomodulatory, anticancer, antiasthmatic, free radical scavenging, antioxidant, analgesic, antiinflammatory, antiulcer, antifertility and wound healing activities (Arulmozhi et al, 2007). Phytoconstituents (three different ingole derivatives) are reported for cytotoxic activity, isolated from the latex of Euphorbia nivulia Buch.-Ham. belongs to Euphorbiaceae family (Veluri et al, 2003). Ethnobotanical values of laticiferous plants used by tribal people of Khandesh region of Maharashtra is recently given by Mahajan and Badgujar, 2008 and they described various traditional (medicinal) applications of some indigenous laticiferous plants. Present investigation is elaboration of previous observations regarding phytochemical examinations of selected twenty one laticiferous plants. MATERIALS AND METHODS Plant material All laticiferous plants were collected in rainy season from Khandesh region of Maharashtra and the corresponding voucher specimen were deposited in the Department of Zoology, Moolji Jaitha College, Jalgaon 425 001, Maharashtra (Alstonia scholaris R. Br. LAT 81, Calotropis gigantea (L.) R.Br. LAT 82, Calotropis procera (Ait.) R.Br. LAT 83, Carica papaya L. LAT 84, Euphorbia hirta L. LAT 85, Euphorbia milii Desmoul. LAT 100, Euphorbia nivulia Buch.-Ham. LAT 87, Euphorbia prunifolia Jacq. LAT 101, Ficus carica L. LAT 90, Ficus hispida L.f. LAT 91, Ficus racemosa L. LAT 92, Ficus religiosa L. LAT 93, Ipomoea carnea Jacq. LAT 95, Manilkara zapota (L.) P. van Royen LAT 97, Pedilanthus tithymaloides (L.) Poit LAT 102, Plumeria rubra L. LAT 103, Plumeria rubra L. forma acuminata (Ait.) Santapau and Irani ex Shah LAT 104, Synadenium grantii Hook. F. LAT 105, Tabernaemontana citrifolia L. LAT 98, Tabernaemontana divaricata (L.) R. Br. LAT 99 and Thevetia peruviana (Pers.) K. Shum. LAT 106) Collection of Latex Latex samples were collected early in the morning from each plant by nipping the leaves near the stem or by incision of the trunk and branches of the plant and allowing the milk to drain in clean glass tube separately, brought to the laboratory and kept in refrigerator (till the experiment start). Latex was homogenized in a homogenizer under chilled condition and filter through four folds of muslein cloth. Filtrate latex samples were used for phytochemical analysis. All necessary chemicals used are AR Grade purchased either from Qualigen fine Chemicals or E. Merck, India. Phytochemical Analysis The different latex samples of Euphorbiaceae, Apocynaceae, Moraceae, Asclepiadaceae, Carricaceae, Sapotaceae and Convolvulaceae families were analyzed for the phytochemical composition by qualitative methods and all latex samples were analyzed for the moisture and total solid content using standard protocols (Kokate, 1994, Harbone1973 and Marinova et al, 2005). Test for alkaloids Three methods were used to test alkaloids. (i) a portion of the latex was treated with few drops of aqueous solution of hydrochloric acid and 0.5 ml Mayer’s reagent. Formation of white precipitate indicates the presence of alkaloid. (ii) few drops of dilute HCL and 0.5 ml Wagner’s reagent has added to a portion of the latex. A brown flocculent precipitate indicates the presence of alkaloid. (iii) a portion of the latex is treated with equimolar mixture of dilute HCL and Dragendorff’s reagent. A brown coloration with precipitate indicates the presence of alkaloid. Test for cynogenic glycosides To 250 µl of the latex was added with equal volume of cold concentrated sulphuric acid. Formation of intense color indicates the presence of glycosides. Test for phenolic compounds Phenolic compounds of latex were detected by Folin Ciocalteu reagent. A portion of the latex was mixed with few drops of diluted Folin Ciocalteu reagent and aqueous sodium carbonate solution. The mixture was allowed to stand for 10 min and formation of gray colour indicates the presence of Phenolic groups. Test for flavonoids Two methods were applied for the qualitative detection of flavonoids. (i) A portion of latex sample was dissolved in 10 % HCL and adds Zinc powder. Appearance of effervescences with pink color indicates the presence of flavonoids. (ii) Latex was dissolved in concentrated H2SO4, formation of intense color observed; this indicates the presence of flavonoids Test for terpenoids A red to purple color formation indicates the presence of terpenoids, when a chloroform soluble portion of latex was treated with an equal volume of concentrated H2SO4. Test for tannins A portion of latex was mixed with few drops of 0.1 % Ferric chloride and observed for brownish green coloration indicates the presence of tannins. Test for saponins To 0.5 ml of latex was dissolved 5 ml of distilled water in a test tube. The solution was shaken vigorously and observed for a stable persistent froth with honeycomb structure indicates the presence of saponins. RESULTS AND DISCUSSION Complete details of identified laticiferous plants with botanical name, family, voucher specimen number, vernacular name and part used as a source of latex is summarized in Table1. Latex obtained from different plant parts of selected laticiferous plants viz., leaves of ten, fruits of six, stem bark of three and couple of whole plants. These belong to the wide group of laticiferous families viz., Euphorbiaceae, Apocynaceae, Moraceae, Asclepiadaceae, Carricaceae, Sapotaceae and Convolvulaceae. The result in Table 2 summarizes the level of moisture and total solid content of individual latex. Highest level of moisture and less content of total solid were found in the latex of E. prunifolia. Lowest level of moisture and highest content of total solid were found in the latex of E. hirta. The Figure 1 illustrates the phytoconstituent wise distribution of laticiferous plant species. The order of secondary metabolites with respect to percentage of latex bearing plants are Phenolics > Alkaloid > Cynogenic glycoside > Tannins> Falvonoids and Saponins >Terpenoids. The result summarizes all latex have phenolic compounds but it was unnoticeable in I. carnea. Terpenoids as seen only in latex of C. papaya and M. zapota. Alkaloid was detected in the sixteen latex samples. Cynogenic glycosides and tannins were detected in eleven and ten number of latex samples respectively. Flavonoids and saponins were detected in equal number of latex samples. The unripe fruit latex of papaya showed positive test towards alkaloids, flavonoids, terpenoids, cynogenic glycosides, phenolics and tannins. Our results are good in agreement of the earlier observation of phytochemical screening of fresh leaves of C. papaya made by Ayoola et al, 2008 and antimalarial activity could be because presence of such diverse group of secondary metabolites. Leafy latex of C. procera showed positive test for alkaloids, phenolics, tannins and saponins and negative observation against test of cynogenic glycosides and flavonoids and our finding is supported by earlier observations made by Hassan et al, 2006 and may be contributed for killing mosquito larvae (Badgujar and Mahajan, 2008 ). Latex sample of A. scholaris showed positive test for alkaloids; Jagetia and Baliga, 2006 explains anticancer activity may be because of alkaloids present in it. The whole plant latex obtained from E. hirta showed presence of alkaloids, phenolics, tannins and flavonoids and absence of terpenoids, cynogenic glycosides and saponins and our observations are in accordance with earlier report of Ogbulie et al, 2005. Leafy latex of I. carnea exhibits the availability of tannins and flavonoids, this plant is commonly employed for wound healing and this confirms earlier claim made by Ambiga et al 2007. The latex of E. nivulia exhibits excellent wound healing activity in rat model at a dose of 500 mg / kg body weight applied topically (Badgujar and Mahajan, 2008) and this activity may be attributed due to presence of glycoside, saponin, alkaloid, phenolics and tannins. Toxicity of T. peruviana is mainly due to the presence of glycosides; such property is confirmed by earlier report of Tanbouly et al, 2000. The architect of cortical cell of the laticiferous tissue is worthwhile to understand in order to locate the exact mechanism of latex secretion. Cortical cell is the origin of secretion of latex from laticiferous tissue. Latex obtained from fruit of Bhuiumber showed presence of alkaloid, glycoside, phenolics compounds and tannins while fruit latex of Umber showed presence of only phenolics compounds and tannins and absence of alkaloid and glycoside. The source of latex was same i.e. unripe fruit and though both are members of Moraceae, however, they exhibit differences in phytoconstituents. It indicates the anatomy of laticiferous tissues is species specific. On the basis of generated information from the present study, it may lead to development of potential bio-product in the treatment of different human ailments and could be exploited in the management of various disease ailments. We think the list of laticiferous plants along with their phytochemical analyses, presented in this study is useful to researchers and in industry regarding pharmaceuticals. Further our intention of this study is to correlate relationship of secondary metabolites to possible biological activities. ACKNOWLEDGEMENTS The authors express thanks to Dr. D. A. Patil, Department of Botany, SSVP’s, Dr. P. R. Ghogray Science College, Dhule and Dr. G. S. Chaudhari, Department of Botany, M. J. College, Jalgaon who helped us wholeheartedly to identify the plants. We are also thankful to Principal of M. J. College, Jalgaon and Principal of C.O.E.T., Bambhori, Jalgaon for providing necessary laboratory facilities to carry out the present work.
REFERENCES 1. Ambiga, S., Narayanan, R., Gowri, D., Sukumar, d. and Madhavan, S., (2007). Evaluation of wound healing activity of Ipomoea carnea Jacq. Ancient science of Life. 26 (3&4): 45 – 51. 2. Arulmozhi, S., Mazumder, P. M., Purnima, A. and Narayanan, L. S., (2007). Pharmacological activities of Alstonia scholaris Linn (Apocynaceae) – A Review. Pharmacognosy Review. 1 (7): 163- 170. 3. Ayoola, G. A., Coker, H. A. B., Adesegun, S. A., Adepoju-Bello, A. A., Obaweya, K., Ezennia, E. C. and Atangbayila, T. O., (2008). Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Tropical Journal of Pharmaceutical Research. 7 (3): 1019 – 1024. 4. Badgujar, S. B. and Mahajan, R. T., (2008). (Unpublished Result). 5. El Tanbouly, N. D., Islam, W. D., Shetata, K. B., Tachibana, Y. and Lee, K. H., (2000). Cytotoxic cardiac glycosides from Thevetia neriifolia Juss roots. Bulletin of the Faculty of Pharmacy, Cairo University. 38: 103 – 106. 6. Harbone, J. B., (1973). Phytochemical Methods: A Guide to modern techniques of plant analysis. Chapman and Hall Ltd., London. 49 – 188. 7. Hassan, S. W., Bilbis, F. L., Ladan, M. J., Umar, R. A. and Dangoggo, S. M., (2006). Evaluation of antifungal activity and phytochemical analysis of stembark extract of Calotropis procera (Asclepiadaceae). Pakistan Journal of Biological Sciences. 9 (14): 2624 – 2629. 8. Jagetia, G.C. and Baliga, M.S., (2006). Evaluation of anticancer activity of the alkaloid fraction of Alstonia scholaris (Sapthaparna) in vitro and in vivo. Phytotherapy Research. 20(2): 103-109. 9. Jaiprakash, B., Chandramohan, D. and Reddy, N., (2006). Burn wound healing activity of Euphorbia hirta. Ancient science of Life. 25 (3&4): 1 – 3. 10. Kokate, C. K., (1994). Practical Pharmacognosy, Vallabh Prakashan, New Delhi. 107– 125, 11. Mahajan, R. T. and Badgujar, S. B., (2008). Ethnomedicinal values of Laticiferous plants used by tribal people of North Maharashtra, India. Research Link. . 55, VII (8): 20-25. 12. Marinova, D., Ribarova, F. and Atanassova, M., (2005). Total phenolics and total flavonoids in bulgarian fruits and vegetables, Journal of the University of Chemical Technology and Metallurgy, 40, (3): 255-260. 13. Nadkarni, A. K., (1976). Dr. M. Nadkarni’s Indian Materia Medica. Popular Prakashan Pvt Ltd, Mumbai. 14. Nath, L.K. and Dutta, S. K., (1992). Wound healing response of the proteolytic enzyme curcain. Indian Journal of Pharmacology. 24: 114-115. 15. Ogbulie, J. N., Ogueke, C. C., Okoli, I. C. and Anyanwa, B. N., (2007). Antibacterial activities and toxicological potential of crude ethanolic extracts of Euphorbia hirta. African Journal of Biotechnology. 6 (13): 1544 – 1548. 16. Pandey, B. P., (2001). Plant Anatomy. (6th revised ed.). S. Chand and Company Ltd., New Delhi. 57-58. 17. Shivkar, Y. M. and Kumar, V. L., (2003). Antihelmintic activity of latex of Calotropis procera. Pharmaceutical Biology. 41 (4): 263 – 265. 18. The Wealth of India (1948): A Dictionary of Raw Material and Industrial Products-Raw Material Series, Vol. I-IX. Publication and Information Directorate, CSIR, New Delhi, India. 19. Veluri, R., Reddy, V.L.N., Reddy, A. V., Kodela, R., Tadikamalla, P. R., Tejomoorty, S. R., Kondapi, A. K., Diwan, P.V. and Yenamandra, V., (2003). Three New Ingol Diterpenes from Euphorbia nivulia: Evaluation of Cytotoxic Activity. Chem. Pharm. Bull. 51(4): 431 - 434.
Table 1. Phytochemical analysis of laticiferous plants undertaken for investigation.
+ = Present, - = Absent, *Part used: LF: Leaf, FT: Fruit, WP: Whole plant, SB: Stem bark
Table 2. Percentage of Moisture and Total Solid Content of Latex .
Figure: 1 Distribution of diverse group of secondary metabolites in laticiferous plants
|