|
Ethnobotanical Leaflets 12: 570-76. 2008.
Antimicrobial and Cytotoxic Activities of Hopea utilis Fruits
M. Maridass
Environmental Carcinogenesis Research Unit St. Xavier’s College (Autonomous), Palayamkottai - 627 002 Tamil Nadu, South India Corresponding Email address: [email protected]
Issued 11 August 2008
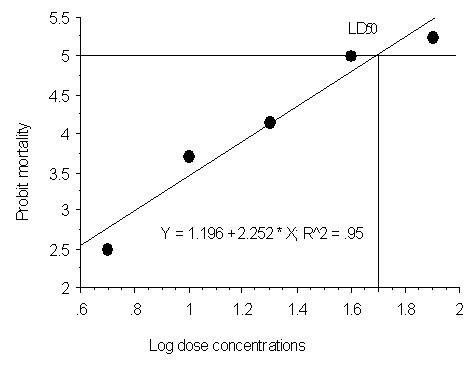
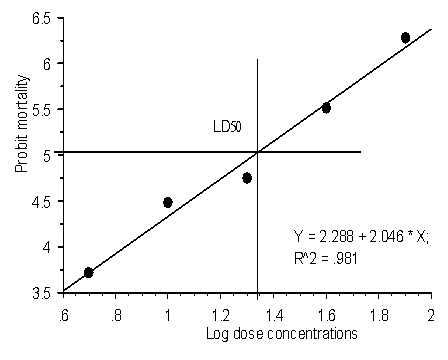
Abstract Aqueous and ethanolic crude extracts of Hopea utilis screened for antibacterial and cytotoxic activities were studied. Antibacterial activity of ethanolic extracts of H. utilis were more successful with the pathogens Salmonella typhi and Streptococcus aureus. The MICs values of ethanolic extract of were Hopea utilis active against Salmonella typhi (25mg/ml), Salmonella typhi (25mg/ml), and Staphylococcus aureus (36mg/ml) respectively. The results of both extracts of aqueous and ethanolic of Hopea utilis showed the brine shrimp lethality assay LD50 values were 1.64μg/ml and 1.34μg/ml. Key Words: Hopea utilis; fruits; antibacterial activity; ethanolic extract. Introduction Plants are recognized for their ability to produce a wealth of phytochemicals. Humankind has for centuries used many species to treat several diseases (Cragg et al., 1999). Recently, a number of tribal studies concerning the search for new antimicrobial agents from plants and antimicrobial screening of the extracts have been published. A rapid and inexpensive test, brine shrimp (Artemia salina) (BST), has been used for screening of biological and cytotoxity activities (De Rosa et al., 1994). The fractions or active compounds in this assay, are further tested in cultured tumoral cells, antimicrobial and antiparasitic assays, generally with good correlation (Sahpaz et al., 1994; Colman-Saizarbitoria et al., 1995; Siqueira et al., 1998). The Dipterocarpaceae is a plant family of 14 genera 750 species found throughout the tropical and temperate regions of the world. Bandaranayake et al., (1977) reported that 44 of the 45 species are endemic. They belong to the genera Cotylelobium, Hopea, Dipterocarous, Shorea, Stemonoporus, Vateria and Vatica. Hopea utilis is a large sized tree distributed in evergreen forest, Southern Western Ghats region, up to sea level 5000 meters. Hopea utilis is locally known as ‘‘Karapongu’’ in Karaiyar region. Ethnobotanical information gathered from tribe of Kanis used as fruits was boiled with water and treatment of stomach pain and hypertension. Bioactive constituents of hopeafuran and C-glycosyl resveratrol were isolated from the stem wood (Tanaka et al., 2001). In the present study, the in - vitro antibacterial and cytotoxic activities of aqueous and ethanol extract of Hopea utilis fruits were investigated. Materials and Methods Collection of plant materials The fruits materials of H. utilis were collected in the Karaiyar, Tirunelveli District, South India and was identified by Dr. U. Manikandan, SPKCES, Alwarkurichi, South India. Extract preparation 250gm of powdered materials was individually extracted with 95% ethanol and water at room temperature. The solvent extract was removed by distillation method. The resulting crude extracts were stored at -20°C until assayed. Antibacterial screening Test Organisms Bacillus subtilis, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pyogenes, Vibrio paarahaemolyticus and Salmonella typhi were studied. Inocula Inocula for the bioassays were prepared by diluting scraped cell mass in 0.85% NaCl solution, adjusted to McFarland scale 0.5 and confirmed by spectrophotometrical reading at 580nm. Cell suspensions were finally diluted to 104 UFC/mL-1 for being used. Disk- diffusion method In - vitro antibacterial activity of both extract, were studied against four gram - positive and two gram - negative bacterial strains by the standard disc-diffusion method (Barry , 1980; Buer et al., 1966; Berghe and Vlientinck, 1991). Nutrient agar was the bacteriological medium. Both extracts were screened at a concentration of 100µg /ml. Diameters of zones of inhibition produced by the isolated agent were compared with those produced by the standard antibiotic (Kanamycin, 30µg /disc ). Determination of MIC and MBC Minimal inhibitory concentration (MIC) and Minimal bactericidal concentration (MBC) were determined for the extracts detailed methods (Suffredini et al., 2004) Cytotoxic activity The cytotoxic effect of both, aqueous and ethanolic extract was evaluated by LC50 of brine shrimp lethality test were followed by Mayer et al., 1982 and Persoone, 1980. Both aqueous and ethanolic extract were dissolved in dimethylsulphoxide (DMSO) separately and five graded doses 5, 10, 20, 40 and 80 mg ml/L were used for 5ml sea water containing 10 brine shrimp nauplii in each group. Mortality was recorded 12h and dead animals were removed immediately. LC50 values, upper and lower confidence limits and slope values were calculated using the POLO computer software (Russell et al., 1979). The regression coefficient between exposure time and different values of LD50 was determined by probit analysis (Finney, 1947). All computations were performed with a computer with a capacity of 28GB in the hard drive, 256 MB in RAM and working with a Microsoft Window XPTM system at a speed of 1.6GHz. RESULTS Many microorganisms which cause damage to human health exhibit drug resistance due to inadequate use of antibiotics. Thus, there is a need for the discovery of new substances from natural resources, including plants (Sartoratto et al., 2004). In the present study, the antibacterial activity of aqueous and ethanolic extract of H. utilis is shown in Table 1. Antibacterial activity of ethanolic extract of H. utilis was more activity observed both pathogen of Salmonella typhi (30mm) and Streptococcus aureus (29mm). The MICs values of ethanol extract active against Salmonella typhi 25mg/ml, and Staphylococcus aureus 36mg/ml, respectively. The cytotoxicity of the compound was bioassayed against brine shrimp nauplii and the results were shown in Table 2. The 50% mortality of log-dose concentrations (LD50) of the aqueous extract was 1.62 µg/ ml. Figure- 1 shown the 95% confidence regression value Y=3.67+1.44X and significant level of (P< 0.05) were observed in aqueous extract of H. utilis. While the more cytotoxic activity was observed in 95% ethanol extract observed seen in Table-2 and Fig.2. The present study was agree to previous workers studied in bioactive compounds of Kolavenic acid and Clerodane diterpine (Islam et al., 2001); Isoflavone (Shah Alam Bhuyan et al., 2003); Triterpenoid (Rahman et al., 2002) and galic acid (Saker et al., 1998). The antibacterial and cytotoxic activity of extract of H. utilis bioassay-guided fractionation procedure to characterize and isolate the bioactive principle is under way in our laboratory. Acknowledgements The author is grateful to Principal, St.Xavier’s College (Autonomous), Palyamkottai-627002, providing for laboratory facilities. REFERENCES Ahmad, I.; Beg, A.Z. Antimicrobial and phytochemical studies on 45 Indian plants against multi-drug resistant human pathogens. J. Etnopharmacol., 74:113-123, 2001. Ali-Shtayeh, M.S.A.; Yaghmour, R.M-R.A.; Faidi, Y.R.B.; Salem K.; Al-Nuri, M.A.D. Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. J. Ethnopharmacol., 60:3: 265- 271, 1998. Baba-Moussa, F.; Akpagana, K.; Bouchet, P. Antifungal activities of seven West African Combretaceae used in traditional medicine. J. Etnopharmacol., 66:3:335-338, 1999. Lentz, D.L.; Clark, A.M.; Hufford, C.D.; Meurer-Grimes, B.; Passreiter, C.M.D.; Cordero, J.; Ibrahimi, O.; Okunade, A.L. Antimicrobial properties of Honduran medicinal plants. Short communication. J. Ethnopharmacol., 63:253-263, 1998. Cragg GM, Boyd MR, Khanna R, Kneller R, Mays TD, Mazan KD, Newman DJ, Sausville EA 1999. International collaboration in drug discovery and development: the NCI experience. Pure Appl Chem 71: 1619-1633. Mahasneh, A.M.A.; Adel, M.A.; El-Oqlah, A.A.B. Antimicrobial activity of extracts of herbal plants used in the traditional medicine of Jordan. J. Ethnopharmacol., 64:271-276, 1999. Martínez, M.J.; Betancourt, J.; Alonso-González, N.; Jauregui, A. Screening of some Cuban medicinal plants for antimicrobial activity. J. Ethnopharmacol., 52:171-174, 1996. Panizzi, L.; Flamini, G.; Cioni, P.L.; Morelli, I. Composition and antimicrobial properties of essential oils of 4 mediterranean Lamiaceae. J. Ethnopharmacol., 39:167-170, 1993. Russell, R.M., Robertson, J.L., and Savin, N.E. (1977). POLO: A new computer programme for probit analysis. Bulletin of the Entomological Society of America, 23: 209-213. Sartoratto,A., Machado,A.L.M., Delarmelina,C., Figueira,G.M., Cristina,M., Duarte,T., Rehder,V.L.G.2004. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Brazilian Journal of Microbiology, 35 : 275-280 Singh, D.K., and Agarwal, R.A. (1984). Correlation of the anticholinesterase and molluscicidal activity of the latex of Euphorbia royleana Bioss. on Lymnaea acuminata. Journal of Natural Products, 47: 702-705. Sokal, R.R.,and Rohlf, F.J. (1973). Introduction to Biostatistics. W.H. Freeman and Co., San Francisco, 271-273.
Table 1. Antibacterial activity of ethanolic and aqueous extract of H. utilis fruits.
Table 2. Antibacterial activity of ethanolic and aqueous extract of H. utilis fruits.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||