|
Ethnobotanical Leaflets 12: 139-149. 2008.
Epidermal Features and Petiolar Anatomy of Angiopteris evecta (Forst.) Hoffm. (Marattiaceae: Pteridophyta)
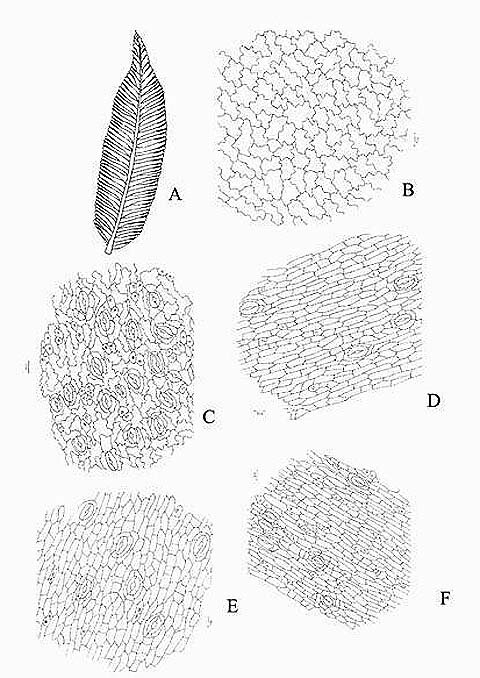
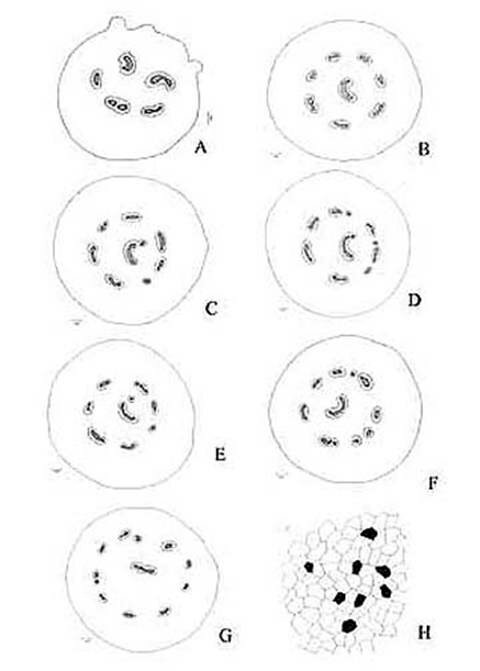
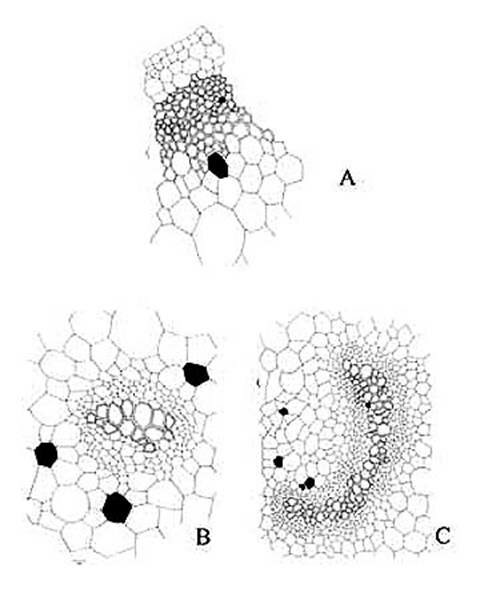
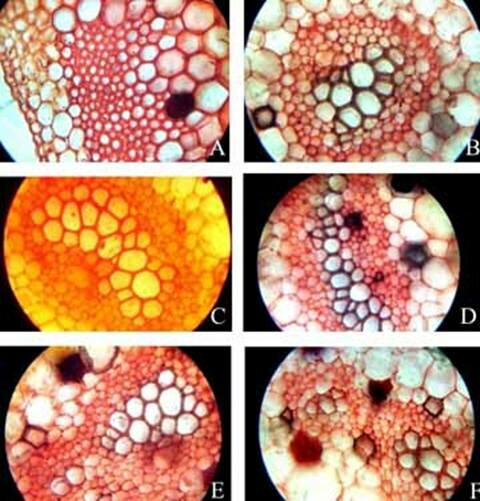
KAMINI SRIVASTAVA Department of Botany, University of Allahabad, Allahabad-211002 India E-mail: Issued 8 March 2008 Abstract Epidermal features and petiolar anatomy of a known ethnomedicinal tree fern, Angiopteris evecta, were studied. The stipe receives eleven separate vascular strands from the rhizome which fuse together to form five strands during their upward course. Key words: Angiopteris evecta, epidermal features, petiolar anatomy, vascular strands. Introduction In the field of vascular anatomy of ferns it is necessary to study the successive stages through which the fern passes during its development. This procedure not only determines the position, interrelationship and structure of the different vascular components of the mature fern but also shows indications of phylogenetic relationship. The epidermal features of pinnae of different Pteridophytes have been studied in the past by Porsch (1905), Kondo (1962), Kondo and Toda (1965), Maroti (1958, 1961), Thurston (1969), Probst (1971) and others. Likewise the stipe characters which too have proved to be of great value (Tansley (1907, 1908), Sinnott (1911), Bower (1914, 1926), Kato (1972), Ogura (1972), Lucansky and White (1974), Linn and Devol (1977, 1978), Khare(1984), etc.) still remain to be studied in case of a number of fern species. With the same view point the epidermal features of pinnae and the exomorphic and internal details of the stipe of Angiopteris evecta have been investigated. Materials and Methods Plant materials of Angiopteris evecta for the present epidermal and anatomical investigations were obtained from the fern house of Botany Department of Allahabad University, India. The plants of this species were originally brought from the forests at Pachmarhi, Madhya Pradesh and the kushmi forest in Gorakhpur district, Uttar Pradesh, India. Pieces of young as well as mature pinnae were fixed in farmers fluid (ethyl alcohol and acetic acid 3:1) and subsequently stored in 70% ethyl alcohol. Epidermal preparations were made by simply peeling out the epidermis and mounting in safranin glycerine jelly. Epidermal slides were also prepared by macerating pieces of mature pinnae in modified Schulzes fluid, using dilute nitric acid and potassium chlorate and subsequently washing and treating with a dilute solution of ammonia (about 1%). Epidermal strips thus obtained were mounted in safranin-glycerine jelly. Their developmental pattern, venation and general orientation of stomata and epidermal cells were investigated in transparencies made by Fosters technique (Foster, 1966). The pinnae of fresh specimens were cleared in 2.5% aqueous sodium hydroxide solution followed by concentrated chloral hydrate, dehydrated in the usual alcohol series and stained with 1% solution of safranin in equal parts of xylene and absolute ethyl alcohol. The descriptive terminology used is after Pant (1965), Metcalfe & Chalk (1950) and others. Anatomy of petiole was studied in serial microtome sections cut in different planes. For microtomy, small pieces of petioles were fixed and stored in F.A.A. which were later washed thoroughly in tap water and dehydrated in a graded series of tertiary butyl alcohol. Infiltration and embedding of the material was done in E. Merck paraffin wax. Serial microtome sections were cut at 10-15 mm thickness. Wherever necessary hand sections were also cut. These sections were stained in the usual safranin fast green combination and mounted in D.P.X. Nature of various depositions and cell contents was identified by special histochemical tests as suggested by Johansen (1940) and Foster (1941). Presence of cutin was confirmed by the appearance of red colour when fresh sections were treated with saturated solution of Sudan IV prepared in 70% ethanol (Margolena, 1932). Presence of starch grains were detected by appearance of blue colour when treated with potassium iodide solution, which was made by dissolving 3 gm of iodine and 1.5 gm of potassium iodide in 100 ml of water. Lignin was tested by occurrence of red colour after treating lignified portion with phloroglucinol solution followed by 1-2 drops of 2.5% hydrochloric acid. Phloroglucinol solution was prepared by dissolving 1 gm phloroglucinol in 100 ml of 95% ethanol. To test the presence of tannin substances fresh sections were placed in 10% aqueous feric chloride solution with a pinch of sodium carbonate and blue colour of tannin substances was observed under the microscope. The presence of phlobaphene was detected by their natural brown colour as suggested by Reeve (1951). Observations Epidermis of pinnae The venation pattern of lamina is open dichotomous and a prominent mid vein is differentiated in the pinnae. The free ends of the veins are often swollen. Vein areas are devoid of stomata (Fig. 1A). The pinnae of Angiopteris evecta are hypostomatic i.e. having stomata only on the lower surface of the pinnae. Epidermal cells on both the faces of pinnae are sinuous walled and irregularly arranged. Near the margin they are less sinuous. The epidermis shows irregularly scattered groups of short, almost straight walled silica containing cells on their lower side. The number of silica containing cells can vary from 1 to 8. The stomata in pinnae are usually placed in the direction of the veins. Stomata are usually amphicyclic showing a ring of four or more clearly differentiated subsidiary cells are one or more rings of encircling cells. Mature guard cells are kidney shaped or slightly rectangular (Figs. 1B, C). The stomatal characters and frequencies of pinnae are mentioned in tables 1& 2. Epidermis of petiole In petiolar portion stomata are irregularly arranged. They are parallel to the surface cells. Each stoma is surrounded by four to six subsidiary cells. One or two cells of silica are also present. Epidermal cells are hexagonal in shape. Mature guard cells are kidney shaped or slightly rectangular (Fig. 1D) (Table 3). Epidermis of rachis The structures of epidermis of both primary and secondary rachis are the same. Both the primary and secondary rachis show irregularly arranged stomata. They are also parallel to the surface cells. Each stoma is surrounded by four to six subsidiary cells. One or two cells of silica are also present. Epidermal cells are hexagonal in shape like those of petiole. Mature guard cells are kidney shaped or slightly rectangular (Fig.1E, F). Pulvinus The pulviuns of Angiopteris evecta is devoid of stomata. It consists of three types of parenchymatous cells. Outer cells are short with flat end walls. Middle region consists of cells which are narrow and elongated with tapering ends and the inner region consists of cells with comparatively thinner walls. Some of the cells contain tannin (Fig. 2H). Anatomy of petiole Transverse section of petiole shows single layered epidermis which consists of thin walled cells. The bulk of petiole is composed of ground tissue. It is differentiated into three zones. The outer most zones consist of 3-4 layers of cells which are made up of thin walled parenchymatous cells. The middle zone consists of 3-4 layers of cells made up of thick walled sclerenchymatous cells, being comparatively smaller in size than the cells of outer and inner zone (Plate1A; Fig.3A). Inner zone consists of large, thin walled polygonal cells filled with starch grains. Starch grains are usually large and spherical or oval in shape. The concentrations of these grains are more towards the base of petiole and gradually decrease towards the apex. At the top of the petiole and in the rachis cells are usually devoid of starch grains. Some of the cells of middle and inner zone contain tannin. Eleven widely separated vascular strands are present at the base of the petiole embedded in the parenchymatous ground tissue (Fig. 2G). Each strand has a single layered endodermis. Endodermis is followed by pericycle containing thin walled cells, which are 1-3 layers in thickness. Xylem lies in the centre of the vascular strand. It is plate like with several protoxylem points in exarch condition. Xylem is surrounded by phloem (Plate 1B, C, D). Phloem consists of sieve cells and parenchyma and xylem has simple tracheids of various sizes. Metaxylem tracheids have scalariform and pitted thickening while protoxylem tracheids have annular and spiral thickening (Fig.3B). The vascular strands in the petiole during their upward course gradually fuse with each other (Plates 1E, F; 2A, B, C, D,) and at last become five vascular strands at the tip. During the fusion, first the endodermis and at slightly higher level the pericycle and ultimately the phloem and xylem bundles of the two strands also fuse together. At this stage vascular strand becomes somewhat C shaped. (Plate2E, F; Figs.2A, B, C, D, E, F, G; 3C). Discussions and Results Angiopteris evecta (Forsk.) Hoffam. is a threatened species which is included in the endangered categories in theRed Data Book of International union for conservation of Nature and Natural Resources. Because of this it has become necessary to study about all parts of this plant. The study of the petiolar anatomy reveals their taxonomic significance. This study also reveals the vascular supply to the petiole with respect to the number of leaf traces arising from the stem stele and entering the petiole and whether they anastomoised, divided or remain unchanged and discussed their bearing on the interrelationships of various taxa. Although there are about 100 species of Angiopteris distributed all over the world, the genus is represented in India by a single species, Angiopteris evecta. It is a robust fern with a globose upright rhizome with considerably thickened roots and spirally arranged leaves. A conspicuous pulvinus is present at the base of pinna. It is usually found in moist shady and humid places along the water streams. The pinnae are hypostomatic. Epidermal cells on both the faces of pinnae are sinuous and irregularly arranged. Near the margin they are more or less sinuous. The epidermis shows irregularly scattered groups of short, almost straight walled silica containing cells in their lower side. The number of silica containing cells can vary from 1 to 8. The stomata in pinnae are usually placed in the direction of the veins. Stomata are amphicyclic showing a ring of four or more subsidiary cells. Petiole is largely made up of parenchymatous tissue field with starch. Usually eleven widely separated vascular strands are present at the base of the petiole embedded in the parenchymatous ground tissue. These vascular strands gradually fuse together during their upward course and ultimately at the base of rachis there remain five vascular strands. Xylem lies in the centre of the vascular strand. It is plate like with several protoxylem points in exarch condition. Xylem is surrounded by phloem. Phloem consists of sieve cells and parenchyma and xylem has simple tracheids of various sizes. Metaxylem tracheids have scalariform and pitted thickening while protoxylem trachieds have annular and spiral thickening.
Fig. 1. Angiopteris evecta. A. A portion of pinna showing venation; B. A portion of upper epidermis; C. Lower epidermis of pinna showing silica bodies; D. Epidermis of petiole showing contiguous stomata and silica bodies; E. Epidermis of primary rachis showing silica bodies and stomata; and, F. Epidermis of secondary rachis showing silica bodies and stomata.
Fig. 2. Angiopteris evecta. A. Diagrammatic transection of petiole at the base showing five vascular strands; B. Diagrammatic transection of petiole a little above the base showing eight vascular strands; C. Diagrammatic transection of petiole more above the base showing eight vascular strands; D. Diagrammatic transection of petiole at its middle portion showing nine vascular strands; E. Diagrammatic transection of petiole a little above its middle portion showing nine vascular strands; F. Diagrammatic transection of petiole more above its middle portion showing ten vascular strands; G. Diagrammatic transection of petiole at its tip showing eleven vascular strands; and, H. Epidermis of pulvinus showing tannin cells.
Fig. 3. Angiopteris evecta. A . A portion of transection of petiole showing epidermis and ground tissue; B. Structural details of a vascular strand; and, C. Structural details of a C shaped vascular strand.
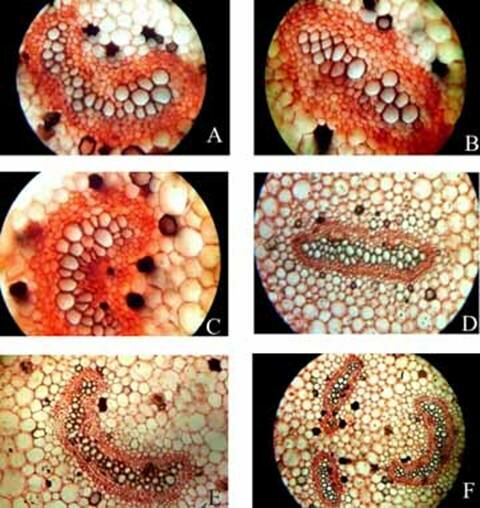
Plate 1. Angiopteris evecta. A. Transection of a portion of petiole showing epidermal cells and ground cells; and, B-F. Transection of petiole showing different types of structural details of vascular bundle.
Plate 2. Angiopteris evecta. A-F. Transection of petiole showing different types of structural details of vascular bundle.
Table1. Epidermal characters of A. evecta.
Table 2. Size and numbers of stomata and epidermal cells of A. evecta.
Table3. Size of Stomata and epidermal cells of petiole in A. evecta.
References BOWER, F. O.1914. Studies in the phylogeny of the filicales.1v. Blechnum and allied genera. Ann Bot. 28:363-431. BOWER, F. O.1926. The Ferns. vol.II. London. FOSTER, A.S. 1941. Practical Plant Anatomy, New York FOSTER, A.S. 1966. Morphology of anstoses in the dichotomous venation of cercaeaster. Amer. J. Bot. 53: 588-599. JOHANSEN, D.A. 1940. Plant Microtechnique. Mc Graw Hill Co. New York. KATO, M. 1972. The vascular structure and its taxonomic significance in the Athyriaceae. Acta Phytotax. Geobot. 25: 79-91. KHARE, P. K.1984 . On the stipe of Adiantum capillus veneris and A.venustum. Developmental and Comparative Aspects of Plant Structure and Fuction. 21-25. KONDO, T. & TODA, H. 1956. A contribution to the study of fern stomata (I), with special references to their development and structure. Res. Bull. Fac. Edu. Shizuoka Univ. 5: 60-80. KONDO, T. 1962. A contribution to the study of fern stomata. Bull. fac. Edu. Shizuoka Univ., 13: 239-67. LIN, B.L. & DEVOL, C.E. 1977. The use of stipe characters in fern taxonomy I. Taiwania. 22: 91-99. LIN, B.L. & DEVOL, C.E. 1978. The use of stipe characters in fern taxonomy II. Taiwania. 23: 77-95. LUCANSKY, T.W. & WHITE, R.A. 1974. Comparative studies of the nodal and vascular anatomy in the neotropical Cyatheaceae, 3. Nodal and petiole patterns; Summary and conclusions. Amer. J. Bot. 61: 818-828. MARGOLENA, L.A. 1932. Fuelgens reaction and some of its applications for botanical material. Stain Technol. 7: 9-16. MAROTI, I. 1958. Untersuchung der Epidermis von pteridophyta Blatt mit besonderer Rucksicht auf die einheimischen Arten. Acta Biol. Szeged. 4: 157-163. MAROTI, I. 1961. Untersuchung der Entwicklung der. Epidermis des Psilotinae und des Filicinae-Blattes und der Entwick lung des stomas Acta.Biol. Szeged. 7: 43-67. METCHALFE, C.R. & CHALK, L. 1950. Anatomy of Dicotyledons. Vol. I, Oxford. OGURA, Y.O. 1972. Comparative Anatomy of the Vegetative Organs of the Pteridophytes, Berlin, Stuttgart PANT, D.D. 1965. On the ontogeny of stomata and their homologous structure. Plant Sci. Ser. 1:1-24. PORSCH, O. 1905. Der Spaltoffnungsapparat im Lichte der Phylogenic. Jena PROBST, W. 1971. Vergleichende Morpholoic und Entwicklungsgeschichte der spaltoffnungenbei Farnen. Stuttgart. REEVE, R.M. 1951. Histochemical test for polyphenols in plants. Stain Technol. 26: 91-96. SINNOT, E.W. 1911. The evolution of the filicinean leaf trace. Ann. Bot. 25: 167-191. TANSLEY, A. G. 1907-1908. Lectures on the evolution of the filicinean vascular system, New Phytol. 6: 25-35, 53-68, 109-120, 135-147, 148-155, 187-203, 219-238, 253-269; 7: 1-16,29-40. THURSTON, E.L. 1969. Taxonomic significance of stomatal patterns in the ferns. Am. Fern. Jour. 59: 68-79.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||