|
Ethnobotanical Leaflets 14: 248-58, 2010.
Phytochemical Screening of Methanolic Extract and Antibacterial Activity of Active Principles of Hepatoprotective Herb, Eclipta alba
Sunita Dalala*, Sudhir K Katariab, KV Sastryb and SVS Ranac
aDepartment of Biotechnology, Kurukshetra University, Kurukshetra-136 119, Haryana, India bDepartment of Zoology(Bioscience), Maharshi Dayanand University, Rohtak-124 001, Haryana, India cDepartment of Environmental Sciences, CCS Meerut University, Meerut, Uttar Pradesh, India *Corresponding author:
Issued: March 01, 2010
Abstract Aerial parts of Eclipta alba are used traditionally for the treatment of several diseases of liver, skin and stomach. Methanolic extract and active principle compound of a well known Indian hepatoprotective herb, Eclipta alba was tested for in vitro antimicrobial studies. It was evaluated using zone of inhibition studies and minimum inhibitory concentration. The extract exhibited activity against all six strains studied. Phytochemical screening of the extract revealed the presence of tannins, flavonoids, coumestans, saponins and alkaloids etc. Ethylacetate fraction and further pure isolated wedelolactone showed enhanced antimicrobial activity. Staphylococcus epidermidis, Staphylococcus aureus and Salmonella typhimurium were most susceptible. Shigella flexneri was the most resistant bacterial strain. These results suggest coumestans/wedelolactone as a promising antimicrobial agent.
Key words: Eclipta alba, wedelolactone, antimicrobial activity.
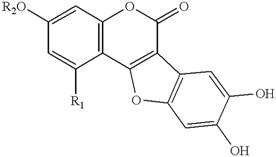
Introduction Modern medicine has evolved from folk medicine and traditional system only after thorough chemical and pharmaceutical screening. The use of synthetic compounds led to a decline in the use of plants in modern medicine. However, synthetic medicine can cause side effects and as a result people are more favorable to use natural compounds obtained from plants. Thus, plants remain a major source of medicinal compounds. About 20,000 plant species are used for medicinal purposes (Penos, 1983). Seventy four percent of 119 plant-derived drugs were discovered as a result of chemical studies to isolate the active substances responsible for their traditional use (Farnsworth and Soejarto, 1991). So plants, especially the higher plants contain a variety of substances, which are useful as food additives, perfumes, and in treatment of various diseases as medicine due to their versatile therapeutic potential (Mukherjee and Wahile, 2006). The active secondary metabolites possess various medicinal applications as drugs or as model compounds for drug synthesis. Phytochemical analysis of plants, used in folklore has yielded a number of compounds with various pharmacological activities. In view of the increasing development of resistant microorganisms, treatment of various diseases caused by microorganisms has become a major challenge in the human medical field. This may be due on the one hand, to the synthetic nature of these substances, but also to their known side effects and in some cases to their unpleasant smell, taste or the burning sensation felt on the skin. Medicinal plants are important substances for the study of their traditional uses through the verification of pharmacological effects and can be natural composite sources that act as new anti-infectious agents. About 3,000 materials from 2,764 plant species have been screened for their pharmacological and chemotherapeutic properties (Anon, 1988). India, in particular has yielded an incredible array of plant products that have drawn the attention of ethno pharmacologists from around the world. Traditionally used medicinal plants produce a variety of compounds of known therapeutic properties (Iyengar, 1976; Harborne, 1989; Chopra et al., 1992). Various biological activities are possessed by E. alba, such as memory disorders treatment, general tonic, edema, fevers and rheumatic joint pains treatment, digestion, hepatitis, enlarged spleen, antioxidant activity and skin disorders (Chopra et al., 1956; Karnick and Kulkarni, 1990; Karthikumar et al., 2007). Wedelolactone is active principle compound of this liver disorder treating drug (Wagner et al., 1986). It also exhibits Trypsin inhibitory effect (Samiulla et al., 2003; Syed et al., 2003), suppresses LPS-induced caspase-11 expression in cultured cells by directly inhibiting the IKK complex (Kobori et al., 2004), treatment of cirrhosis of the liver and infectious hepatitis (Murphy et al., 1979). The shoot extract of E. alba showed antimicrobial (Anonymous 1952; Kosuge et al., 1985; Wiart et al., 2004), antifungal activity (Venkatesan and Ravi, 2004) and weak cytotoxicity against the M-109 cell lines by alkaloids Verazine (Abdal Kadar et al., 1998), antiviral activity against Ranikhet disease virus (Khin et al., 1978), effective against internal and external parasites (Lans et al., 2001) G. intestinalis (Sawangjaroen et al., 2005), antibacterial (Kumar et al., 2007). Since E. alba is a weed /herb growing in dump, moist puddles distributed in the tropical and subtropical regions of the world. So besides ethnobotanical evidence, it can be hypothesized that plants which survive in media rich in microbes most likely be possessing antimicrobial principles. However, up to date, research has been done to investigate various pharmacological activities and antimicrobial activity of only crude extracts of this traditionally used herb. We report here our findings on antibacterial effects of wedelolactone (Fig. 1), the principle active compound, extracted from E. alba.
Figure 1. Chemical structure of Wedelolactone (R1-OH, R2-CH3).
Materials and MethodsPlants of E. alba were collected locally from botanical garden and surroundings of Maharshi Dayanand University, Rohtak. The plant was duly authenticated and voucher specimens (EA-06/) were deposited in the herbarium section, Dr Jaya Parkash Yadav, of Department of Biosciences, Maharshi Dayanand University, Rohtak (Haryana) India.
Qualitative estimation of primary and secondary plant metabolites All estimations were done following Hunda et al. (1985); Brindha et al. (1981).Different aerial parts of the plant were dried at room temperature, powered, and extracted with methanol (70% v/v) in Soxhlet apparatus for six hours. The extract was filtered and was tested with different alkaloid reagents.
Methanol extract The three months old 950 gm lyophilized leaves were Soxhlet extracted with methanol for 36 h.
Ethyl acetate fraction The methanol was removed from extract and the residues were suspended in water separately and heated on steam bath below 80 C for 30 min. After filtration, the aqueous phase was partitioned with ethyl acetate. The organic phase was dried, filtered and the solvents were evaporated to yield 6.8 gm light brown powder.
Isolation of Wedelolactone The powder was subjected to fractionation by column chromatography on silica gel, eluted with the solvent of increased polarity i.e. Non-polar - polar - highly polar. The coumestans are polar compounds so the solvent combination found suitable for their elution was Chloroform + Methanol (70 + 30). They were eluted simultaneously in 37 to 48 fractions. The pooled sample was then subjected to TLC, the solvent system (Toluene : Acetone : Formic acid :: 11 : 6 : 1 v/v) showed two spots with Rf values 0.39 and 0.28 which matched with the Rf values of reference wedelolactone and demethylwedelolactone respectively (Courtesy M/s Natural Remedies, Bangalore, India). The purified sample of wedelolactone was put to HPLC for further qualitative analysis using instrument - Thermo Finnigan from Thermo Electron Corp. USA, with quaternary pump and online degasser system with Auto sampler equipped with Photo Diode Array (PDA) detector, ChromQuest Version 5.0 for data interpretation and Supleco C8 Discovery column, 15 cm x 4.6 mm, Lot No. 59353 (Fig. 2).
Figure 2. The chromatogram of extract showing the peak.
Preparation of samples for testing The zone of inhibition studies were conducted with various extracts diluted with 10% dimethylsulfoxide (DMSO).
Micro organisms Standardized strains from the American/Microbial type culture collection were used in bioassays. Staphylococcus aureus (ATCC 9144), Salmonella typhimurium (ATCC 23564), Escherichia coli (ATCC 10536), Staphylococcus epidermidis (ATCC 155), Shigella flexneri (ATCC 29508) and Pseudomonas aeruginosa (ATCC 25668) were cultured at 37 C on nutrient medium in aerobic conditions for 24 h. These bacteria were obtained from the Institute of Microbial Technology, Chandigarh. Antimicrobial susceptibility testing MIC of wedelolactone was determined by microdilution technique as described by the National Committee for Clinical Laboratories standards (2000). The MIC was defined as the lowest concentration of the compound to inhibit the growth of microorganism. The bacteria inoculums were prepared in 5 ml nutrient broth and incubated at 37C. The final inoculums were of approximately 5 x 106 CFU/ml. Controls with 0.5 ml of culture medium with out the samples and other without microorganisms were used in the tests. Tubes were incubated at 37C for 24 h. The activity was measured as a function of turbidity at 660 nm. Lack of turbidity was further confirmed by pouring suspension aliquot of 0.1 ml into pre-sterilized Petri dishes with nutrient agar medium. The tests were conducted in triplicate. Agar well diffusion method was carried out by allowing perforation of extract and wedelolactone dissolved in DMSO at a concentration of 3.5mg/well and 10 mg/ml respectively. Petriplate containing 30 ml nutrient agar medium were kept for the solidification before inoculating the microorganism, desired numbers of holes of uniform diameter of 8mm were made after solidification, using sterile aluminum borer. 0.2 ml of compound, positive (Gentamycin) and negative (solvent blank) controls were poured into wells. After incubation for 24 h at 37 C the plates were observed and the compound activity was evaluated by measuring zone of inhibition (diameter mm). The tests were conducted in triplicate. Gentamycin (10.0 g/ml) was used as positive control. The negative control was 10% DMSO. Results The results of the presence of various primary and secondary metabolites in methanol extract (Table 1) reported negative for antraquinones throughout herb. Methanol extract and Ethyl acetate fraction showed positive signs of antimicrobial activity against all six strains (Table 2). Wedelolactone exhibited significant antibacterial activity against the six tested strains (Table 3). S. epidermidis and S aureus were found to be highly sensitive. The MIC of S. epidermidis, S. typhimurium, S. aureus, P. aeruginosa, S. flexneri and E. coli was, 15 g/ml, 25 g/ml, 20g/ml, 1250 g/ml ,1300 g/ml and 1000 g/ml respectively. The compound showed the highest antibacterial activity (ZOI) in S. epidermidis (10.24 mm), followed by S. typhimurium (9.16 mm), S. aureus(9.14 mm),E.coli (8.60mm) P. aeruginosa (8.00 mm) and S. flexneri (7.60 mm) as zone of inhibition in radius.
Table 1. Quantitative estimation of the various primary and secondary metabolites.
Table 2. Antibacterial activity of Methanol extract and Ethyl acetate fraction against bacterial strains.
Discussion The primary and secondary metabolites were analyzed in methanolic extracts. Anthraquinones were found to be absent in the natural plant. While the alkaloids and reducing sugars were absent in root extract of the natural plant. In an earlier study, the extracts of the leaves of E. alba tested positive for steroid, reducing sugars, alkaloids, phenolics, saponins and tannins, but no anthraquinones and flavonoids were detected (Gopalakrishan and Solomon, 1992). In Gujrat and Punjab, E. alba is used externally for ulcers and as an antiseptic for wounds in cattle and is reported to treat many microbial infections in rural areas (Warrier, 1994). The results from the current studies revealed that the wedelolactone may be the main constituent responsible for antimicrobial activity. There are various reports that crude extract from E. alba and E. prostrata showed antibacterial, antifungal and anti viral activity (Kosuge et al., 1985; Wiart et al., 2004; Venkatesan and Ravi, 2004; Khin et al. 1978; Karthikumar et al., 2007). Wedelolactone exhibited effective antibacterial activity against all the six strains studied. It proved highly effective against S. epidermidis and S. typhimurium demonstrating the specificity of wedelolactone activity.
Table 3. Antibacterial activity of wedelolactone and gentamycin against bacterial strains.
MIC: minimum inhibitory concentration; ZOI: zone of inhibition (Diameter); A: wedelolactone (10.0 mg/ml); B: gentamycin (10.0 g/ml); Values are mean of three replicates.
Karthikumar et al., (2007) reported in vitro antimicrobial activities of ethanolic extract of E. prostrate. It indicated good activity against S aureus 7.2mm (ZOI) and MIC 70l/ml, and for P. aeruginosa 8.8mm (ZOI) and MIC 65l/ml, at 50 g concentration. While the present studies exhibited respective ZOI at 9.14 mm, 8.00mm and MIC 20 g/ml and 1250 g/ml respectively. Traditional reports on E. alba indicate that it is one of the herb used for treatment of stomach and digestion disorders, skin diseases and conjunctivitis (Poonam and Singh, 2002).Since S. typhimurium, S.flexneri and S. aureus are pathogens responsible for stomach disorders ,while P.aeruginosa is common in skin flora and S. aureus is responsible for most common bacterial conjunctivitis. The results from the current studies revealed that the wedelolactone could be the main constituent responsible for these treatments as it exhibited good activity against them. Conclusion On the basis of the antibacterial studies of wedelolactone, it can be suggested that this can be used effectively to treat S. epidermidis and S. typhimurium infections. However, the compound must be studied in animal models to determine the efficacy in vivo against these pathogens and to elucidate their mechanism of action. In vivo data may be helpful in determining the real potential usefulness of this plant for the treatment of infectious diseases. AcknowledgementsThe authors wish to thanks Dr. T Velpandian, Associate Professor Ocular Pharmacology Laboratory at All India Institute of Medical Sciences, New Delhi, India for HPLC analysis and the members of laboratory of Department of Biosciences, MD University, Rohtak, Haryana, India. ReferencesAbdal, K.M.S., Malone, B.D.B., Werkhoven, S., Van, M.C., David, T.F., Wisse, J.H., Bursuker, I., Neddermann, K.M., Mamber, S.W., and Kingston, D.G., 1998. DNA damaging steroidal alkaloids from Eclipta alba from the Surinam rainforest. J Natl Prod. 61(10): 1202-1208. Anon, P., 1988. Pharmaceutical and cosmetic compositions containing tomato plant extracts for the treatment of skin diseases. Patent-Israel, 78 (820):15. Anonymous (1952). The Wealth of India, Raw Materials, CSIR New Delhi, 3, 127-128 pp. Brindha, P., Sasikala, B., and Purushothaman, K., 1981. Phytochemical analysis of E. alba. BMEBR. 3(1): 84-96. Chopra, R.N., Nayar, S.L., and Chopra, I.C., 1956. In Glossary of Indian Medicinal Plants. Council of Scientific and Industrial Research, New Delhi India, 104 pp. Chopra, R.N., Nayar, S.L., and Chopra, I.C., 1992. In Glossary of Indian Medicinal Plants. Council of Scientific and Industrial Research New Delhi. 3rd edn., 7-246 pp. Farnsworth, N.R., and Soejarto, D.D., 1991. Global importance of medicinal plants. In: Akerele, O, Heywood, V, Synge, H. (Eds.), The conservation of medicinal plants. Cambridge University Press, Cambridge, UK, pp. 25-51. Harborne, J.B., 1989. Recent advances in chemical ecology. Nat Prod Rep. 8: 85-109. Hunda, S.S., Prakash, P., and Roy, B., 1985. Bioactivity directed extraction and fractionation of E. alba: An hepatoprotective drug of Indian origin. Ind J Pharma Sci. 13: 50-51. Iyengar, M.A., 1976. Bibliography of investigated Indian medicinal plants. (1950-1975). Ist Edn. Manipal College of Pharmacy, Manipal Medical College. Karnick, C.R. and Kulkarni, M., 1990. Ethnobotanical studies of some medicinal plants used in skin diseases. Maharasthra Med J. 37: 131-134. Karthikumar, S., Vigneswari, K., and Jegatheesan, K., 2007. Screening of antibacterial and antioxidant activities of leaves of Eclipta prostrata (L). Scientific Res Essay, 2(4): 101-104. Khin, Ma-Ma., Nayunt, N., and Khin, M.T., 1978. The protective effect of Eclipta alba in CCl4 acute liver damage. Toxico Appl Pharmacol. 45: 723-728. Kobori, M., Yang, Z., Gong, D., Heissmeyer, V., Zhu, H., Jung, Y.K., Gakidis, M.A. , Rao, A., Sekina, K., Ikegami, F., Yuan C., and Yuan, J., 2004. Wedelolactone suppresses LPS-induced caspase 11 expression by directly inhibiting the IKK complex. Cell Death Differentiation. 11(1): 123-130. Kosuge, T., Yokota, M., Sugiyama, K., Yamamoto, T., Ni, M., and Yan, S., 1985. Studies on antitumor activities and antitumor principles of Chinese herbs. I. Antitumor activities of Chinese herbs. Yakugaku Zasshi. 105(8): 791-795. Kumar, G.S., Jayaveera, K.N., Ashok Kumar, Sanjay, C.K., Swamy, B.M.V., and Kumar, D.V.K., 2007. Antimicrobial effect of Indian Medicinal plants against acne-inducing bacteria. Trop J Pharma Res. 6(2): 717-723. Lans, C., Harper, T., Georges, K., and Bridgewater, E., 2001. Medicinal and ethnoveterinary remedies of hunters in Trinidad. BMC Complement Altern. Med. 1: 10. Morales, G., Paredes, A., Sierra, P., and Loyola, L.A., 2008. Antimicrobial activity of three Baccharis species used in the traditional medicine of Northern Chile. Molecules. 13: 790-794. Murphy, R.C., Hammerarstrom, S., and Samuelsson, B., 1979. Leukotriene C: A slow reacting substance from Murine mastocytoma cells. Proc Natl Acad Sci USA, 1976: 4275-4279. Mukherjee, P.K., and Wahile, A., 2006. Integrated approaches towards drug development from Ayurveda and other Indian system of medicines. J Ethanopharmacol. 103: 25-35. National Committee for Clinical Laboratories standards 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standards (M7-A5), Wayne Pa. Poonam, S., and Singh, G., 2002. A review of plant species used to treat conjunctivitis. Phytoterapy Res. 16(1): 122. Penos, G., 1983. Index Plantarum Medicinalium Totius Mundi Eorumque Synonymorum (EMPLED). Org. (Ed.) Med. Fram.Nfilano. pp. 188-195. Ross, I.A., 2001. Medicinal plants of the world. Humana Press, Totowa, New Jersey, Vol II, 225-235 pp. Samiulla, S., Mundkinajeddu, D., Shivanna, Y., Arun, C., Keerthi, M., Prashanth, D., Amit A., and Venkataraman, B.V., 2003. Trypsin inhibitory effect of wedelolactone and demethylwedelolactone. Phytoter Res. 17(4): 420-421. Sawangjaroen, N., Subhadhirasakul, S., Phongpaichit, S., Siripanth, C., Jamjaroen K., and Sawangjaroen, K., 2005. The in vitro antigiardial activity of extracts from plants that are used for self-medication by AIDS patients in southern Thailand. Parasitol Res. 95(1): 17-21. Syed, S.D., Deepak, M., Yogisha, S., Chandrashekar, A.P., Muddarachappa, K.A., DSouza, P., Agarwal, A., and Venkataraman, B.V., 2003. Trypsin inhibitory effect of wedelolactone and demethylwedelolactone. Phytother Res. 17 (4): 420-1. Venkatesan, S., and Ravi, R., 2004. Antifungal activity of Eclipta alba. Indian J Pharamceutical Sci, 97-98. Wagner, H., Geyer, B., Yoshinobu, K., and Govind, S.R., 1986. Coumestans as the Main Active Principles of the Liver Drugs Eclipta alba and Wedelia calendulacea. Planta Medica. 5: 370-2 Warrier, P.K., 1994. Indian medicinal plants. Orient Longman Ltd, Chennai Vol-II, 350 pp. Wiart, C., Mogana, S., Khalifah, S., Mahan, M., Ismail, S., Buckle, M., Narayana A.K., and Sulaiman, M., 2004. Antimicrobial screening of plants used for traditional medicine in the state of Perak, Penisular Malaysia. Fitoteropia. 75 (1): 68-73.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||