THE PRESENCE OF 5-HYDROXY- METHYL-FURFURAL IN THE SHELLS OF THE DURIAN FRUIT (DURIO ZIBETHINUS MURR.) AS MINOR DEODORANT AND ITS POSSIBLE ROLE IN THE RIPENING PROCESS OF THE FRUIT
Oen Liang-Hie and M. Soemartini
Department of Biochemistry, Medical School, University of Indonesia, Jakarta and The Faculty of Mathematic and Natural Science, University of Indonesia, Jakarta, Indonesia
INTRODUCTION
The durian fruit (Durio Zebethinus Murr.) is well-known in several South East Asian countries, such as Thailand, Malaysia, Singapore and Indonesia.
The spiky fruit, can reach the size of a football, when ripe (see picture).
 The durian fruit is quite well-known for its distinctly pervasive and overwhelming aroma. Those who like the fruit would call the taste of the durian meat “heavenly” and they would not mind to go long distances to obtain the delicacy.
The durian fruit is quite well-known for its distinctly pervasive and overwhelming aroma. Those who like the fruit would call the taste of the durian meat “heavenly” and they would not mind to go long distances to obtain the delicacy.
On the other hand those who dislike it call the smell fetid and abhor the fruit.
This paper is targeted to the former group.
The ripe durian in the wild would attract man and animal, such as bats, birds, monkeys and even elephants.
When it reaches full maturity, the fruit will easily open along certain “lines” among the spikes.
The creamy custard textured yellow colored fruit-meat will be seen snugly packed into a cylinder, in a row on both sides of the shell. Inside the fruit-meat are the “stones”, which are the seeds of the durian fruit.
Removing the fruit-meat from the shells, will leave empty “cradles” like grooves.
Durians are usually eaten with the fingers. When you finished eating, you wash your fingers and rinse your mouth to get rid of the durian smell.
However a small but detectable durian smell still cling around the fingers and in the breath.
Many people would just ignore this annoying smell as they consider: this is to be a small price to pay for the joy of eating durian.
And here is where our problem starts.
The well-known and well-believed traditional way to get rid of this annoying smell is as follows: Take a few empty durian shells and fill the hollows with water.
A few back and forth scrapings with your finger across the water in the grooves, would enrich the compound content of the rinsing water thereby improving the deodorizing activity (see picture). Then use this water to wash your fingers and rinse your mouth.

The first author knows about this tradition but could not believe it to be true. So he did the above prescribed maneuvers and to his great surprise the annoying lingering smell vanished.
Realizing that a more objective way was necessary to prove or disprove this phenomenon he set up the following experiments in the laboratory.
THE EXPERIMENTS
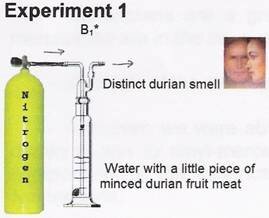
The non-reactive and odorless N2 gas is bubbled through the water containing a small piece of durian and this will carry its smell outside the bottle B1.
* Each B1 is freshly prepared
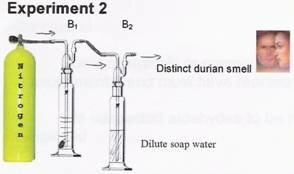
The detectable durian smell at the end of the bottle B2 means that no reaction took place between the durian smell and the soap water.
Experiment 1 was repeated before experiment 3 to ensure that the durian smell was still detectable.
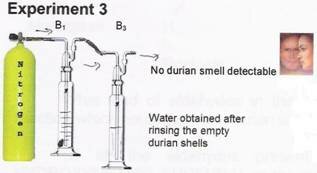
The durian smell was not detectable at bottle B3 after passing through the column of water obtained after rinsing the empty durian shells.
Note:
To reproduce experiment 3, please use only a small piece of durian meat as the deodorizing action of the rinse water contain only a small amount of the compound.
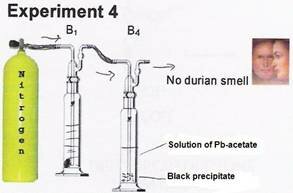
When the durian smell was passed through a solution of Pb-acetate, a black precipitate can be seen at the bottom of bottle 4.
The black precipitate resulted as the mercaptans* present in the durian smell reacted with the Pb.
* Mercaptans are a group of sulfur containing organic chemical substances. If mercaptans are in the air, even at low concentrations, they are very noticeable.
Analysis of the mercaptans present in the durian smell is beyond the scope of our project.
However, we were able to find out that the major part of the “heavenly” smell of the durian is due to amyl-mercaptan. This was revealed to us when we were given the components of the essence of durian smell by Naarden Essence Company in The Netherlands.
It is known that mercaptans with short chains of up to 4 to 6 carbon atoms are volatile and have a pleasant odor.
CONCLUSION
Some chemicals or compounds must have been present in the rinse water bottle in experiment 3 and must have reacted with the mercaptans present in the durian smell.
We suspect aldehydes to be responsible and the following chemical reaction is suggested:
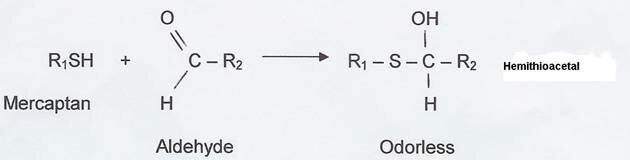
What kind of aldehydes in the shells would be responsible for this chemical reaction which neutralizes the durian smell (although weakly)???
Of all the aldehydes present in the durian plant, we focused on 5-hidroxy-methyl-furfural (5-HMF) as the most possible and the most abundant in nature.
Our choice fell upon 5-HMF as this component is derived from the sugar aldo-hexose which is also abundant in nature (see figure)
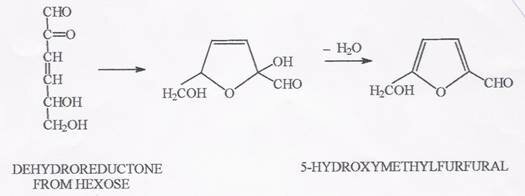
5-Hydroxy-methyl-furfural formation
Note: 5-HMF is a weak deodorant and is present in small amounts in the durian fruit shells (See experiment 3).
Identification of 5-HMF
The phenylhydrazone derivative of HMF were easily obtained after the prescribed procedure. Pale yellow spheres with Melting Point (MP) ± 800 C were obtained after recrystalization.
The dinitrophenylhydrazone derivative of the same compound was obtained after silicagel chromatography as suggested by Shriner (1964).
The results obtained were bright-orange irregular crystals with a MP range of 2250 – 2300 C in ethyl-acetate.
Bright orange of irregular shapes were obtained with an MP range of 2450 – 2500 C in alcohol-water.
We hereby state that the compound present in the water used to rinse the durian shells contain 5-HMF originally present in the durian shells.
The authors believe that the 5-HMF which is present in the shells of the durian fruit do have another more important role, i.e. in the reproductive cycle of the durian fruit.
We believe that the young unripe fruit should be sufficiently protected against too early an attack or assault from those cherishers of the fruit, like bats, monkeys, elephants and probably man himself. If this happens, then the uneaten unripe seeds will not sprout, which means ending the reproductive cycle.
As the ripening process of the fruit continues, more and more durian meat would produce mercaptans, overwhelming the protective coverage of the available 5-HMF.
The ever-increasing strength of smell of the ripening durian finally seeps through the shells and the environment will be filled with it: indicating that the durian fruit is now ripe to be consumed and its seeds ready to sprout.
We believe that the 5-HMF besides its function as a minor deodorant of the durian smell do participate in a bigger role; which is the continuation of the life cycle of the durian fruit.
NB. The authors like to pay homage to our early fore-fathers who have discovered this remedial method, without any reasonable explanation, absent their knowledge of chemistry at that time.
AKNOWLEDGEMENT
The authors are very much indebted to the tire-less support of Dr. Benny Handojo Rahardjo in preparing this manuscript.
NOTE:
A preliminary report of this work was presented at the 5th Southeast Asian and Western Pacific Regional Meeting of Pharmacologist in Beijing, China, July 4 – 8, 1988.
REFERENCES
Cheronis, N.D., Semimicro qualitative organic analysis, 1954, pp. 390 – 593.
Lehninger, A.L., The molecular basis of cells structure and function, Biochemistry, 2nd ed., Worth Publisher, Inc., New York, 1975, page 249 – 255.
Shriner, R.L., Morgan, E.N. and Finlay, W.P.K., Systematic identification of organic compounds, 5th ed., A Wiley International Edition, 1964, pp. 147, 253 – 254.
Plumer, D.T., An introduction to practical biochemistry, 2nd ed., 1979, pp. 143.