Ethnobotanical Leaflets 12: 231-244. 2008.
Phytochemicals From Genus Diospyros (L.) and their Biological Activities
M. Maridass
Animal Health Research Unit
St. Xavier’s College (Autonomous)
Palayamkottai – 627 002, Tamil Nadu, India
E-mail:[email protected], or
Issued 11 May 2008
Abstract
Various constituents isolated and characterized from Diospyros species are described. These include naphthaquinones, triterpenoids and steroids. Some notable activities reported from the various part of the plant and from the extract and isolated constituents are antibacterial, antifungal, antiprotozoal, antimolluscocidal, anti-inflammatory and cytotoxicity activity.
Introduction
Human beings have been influenced in various ways by plants and their products. Plant parts are used in all branches of medicine such as Allopathy, Homeopathy, Unani and the Ayurvedic system. The genus Diospyros L. (Ebenaceae), which is distributed throughout the tropics, is characterized by its ability to produce triterpenes of the lupine series (Mariadel, 1995). The genus Diospyros consists of ca 240 species, 59 of which are distributed in India (Neeru Jain, 1994), Thailand, Japan, Nigeria, South Africa and Philippines.
In the Philippines the tree D. blancoi is commonly found in forest at low altitudes and is planted along the roadsides for shade. In the Philippines, it is called Mabolo Persimmon or Velvet apple. The taxonomic status of this plant is confusing and it has generally been called D. discolor Willd. The tree is also used for timber in the Philippines and, according to Burkill 1966, the best hair combs in the Phillipines are made from it. Diospyros peregrine Gurka (Syn. D. embrypteris Pers; D. malabarica Desr.) is reported to possess many medicinal properties. The plant has an astringent action and is particularly used for the treatment of diarrhoea and dysentery. An ether extract of the fruits possesses antibacterial properties, and has also been used for dye making and tanning fishing nets (Anon, 1952; Walt, 1980).
The leaves of the African medicinal plant, D. leucomelas, were tested and found to contain the triterpene components betulin, betulinic acid and ursolic acid. They showed anti-inflammatory activity in the carrageenan and serotonin, induced paw edema tests and TPA and EPP ear edema tests in mice (Mariadel et al., 1995).
Another species, D. morrisian, known as Shan Hung Shig in the herbal medicine of Taiwan has been claimed to possess antibiotic activity (Wu et al., 1972). The hexane extract of the stem parts of this plant was found to show significant cytotoxicity against in vitro tissue culture cells (Xiu-Zhen Yan et al., 1989). Several Diospyros species such as D. ismailli Ng, D. siamang Bakh., D. wallichii Williams, D. toposoides and D. rufa K & G are reported as being effective for curing skin diseases (Burkill, 1966). The extract of the fresh fruits of D. mollis Griff. are widely used in Thailand as an antihelmintic and a readily oxidizable phenolic component (Loder et al., 1957). Identified from D. usambarensis root bark were the following components: 7-methljuglone, mamegakinone and isodiospyrin, the latter exhibiting mulluscicidal and antifungal properties (Marston et al., 1984). D. tricolor Hiern is used in Nigeria as a chewing stick and in various indigenous formulations for leprosy and dysentery (Heyhauer, 1966; Loder et al., 1957).
The bioactive compounds and biologically active extracts from different Diospyros species have been summarized in Table I and Table II. The chemical constituents isolated from different plant parts of Diospyros species have been given in the Table III.
Table I. Biological activity of different extracts from Diospyros species.
|
S. No |
Plant species |
Different extracts |
Biological activity |
Ref. |
|
1. |
D. tricolor |
Petroleum ether |
Antimicrobial |
Abike et al., 1994. |
|
2. |
D. Montana |
Ethanol |
- |
|
|
3. |
D. marrisiana |
Hexane |
Cytotoxicity Antibiotic activity |
Xiu-Zhen Yan et al., 1989. Wu et al., 1989. |
|
4. |
D. peregrina |
Ethanol |
Antiprotozoal |
Kirtikar et al., 1935. |
|
|
|
|
Antiviral |
|
|
|
|
|
Hypoglycemic Antibacterial |
|
Table II. Biological activity of different compounds from Diospyros species.
|
S. No |
Plant species |
Different compound |
Biological activity |
Ref. |
|
1. |
D. leucomelas |
Betulinic acid |
Anti-inflammatory |
Recio et al., 1995. |
|
|
|
Betuline |
Anti-inflammatory |
|
|
|
|
Ursolic acid |
Anti-inflammatory |
|
|
2. |
D. morrisiana |
Isodiospyrin |
Cytotoxicity |
Xiu-Zhen Yan et al., 1989. |
|
|
|
b-amyrin |
Cytotoxicity |
|
|
|
|
Olean-12-en-3-one |
Cytotoxicity |
|
|
|
|
Bi-Naphthoquinone |
Cytotoxicity |
|
|
3. |
D. tricolor |
Diosquinone |
Antibacterial |
Alake et al., 1994. |
|
4. |
D. mollis |
Phenolic compound |
Anthelmintic |
Loder et al., 1957. |
|
5. |
D. usambarensis |
7-methyljuglone |
Molluscocidal and antifungal |
Marston et al., 1984. |
|
|
|
Mamegakinone |
Molluscocidal and antifungal |
|
|
|
|
Isodiospyrin |
Molluscocidal and antifungal |
Table III. Chemical compounds of Diospyros species.
|
S. No |
Plant species / Plant part |
Compound |
Ref. |
|
1. |
D. peregrine roots |
Dihydroflavonol glycoside 5, 7, 3, 5’ – Tetra hydroxyl – 3’ – methoxy flavone 4’–O- a-L–Rharmnopyranoside |
Chauhan et al., 1979. |
|
|
Leaves |
Triterpenes, anthrocyanin |
Neeru Jain et al., 1994. |
|
|
Fruits |
Lup-20 (29)-3n-3a, 27-diol-29 Lup-20 (29)-3n-3b-diol-29 Taraxerone Sitosterol Gallic acid Peregrinol |
Mishra et al., 1971.
|
|
|
Fruit Pulb |
Hexacosane Hexacosanol b-sitosterol Monohydroxy triterpene ketone Betulin b-D-Glycoside of b–sitosterol Gallic acid |
|
|
|
|
Betulinic acid Methyl ester acetate, Methylester B-D-Glycoside of b-sitosterol |
|
|
2. |
D. mollis Griff Fruits |
Lupeol a-amyrine b-sitosterol Diospyrol 1, 8 dihydroxynapthalene 8-dihydroxy-2-acetyl-3-methyl naphthalene |
Sturm et al., 1971
Yoshihira et al., 1967.
|
|
3. |
D. Montana Leaves |
Lupeul Sitosterol, Stigmasterol, Epi-uvaol, Betulin, Urs-12-en-3b-28-diol Oleanolic acid |
Dutta et al., 1972.
-
|
|
4. |
D. melanoxlon Roxb. (Heart wood) |
b-sitosterol terpenoid Lupeol Betulin Betulinic acid 2-methyl-5-methoxy-1 4-naphthaquinone, 3-methyl-8-methoxy-1, 9, naphthaquinone, 2-methl-3-hydroxy-5-methoxy, and 2-methyl 5, 6 Di methoxy-1, 4-napthaquinone. |
Sidhu et al., 1968.
|
|
|
Leaves |
b-sitosterol Monohydroxy monocarboxylic acid, Monohydroxy triterpene Bauererys acetate, Ursolic, Betulinic acid, Baurenol, ursolic Diospyric acid, Isobanerenol, Methyl betulinate |
Sidhu et al., 1968.
|
|
5. |
D. morrisiana Root Stem |
Isodiospyrin Betulinic acid
Isodiospyrin b-amyrine Olean-12-en-3-one b-amrine acetate |
Xiu-Zhen Yan et al., 1989 Yoshihira et al., 1970. Kuroyanagi et al., 1971. Lee et al., 1984. Xiu-Zhen Yan et al., 1989. |
|
6. |
D. ismailli Ng Fresh wood |
Novel napthoquinone Coumarin Ismallin 4-hydroxy-5-methyl coumarins 4-hdroxy-5-methy |
Jeffreys et al., 1985.
Zakaria et al., 1989. |
|
7. |
D. lotus (L.) |
Taraxerol, Isodiospyrin, 7-methyljugulone Betulinic acid Xallobetulin 8, 8’-dihydroxy-6, 61-dimethl binaphtho quinonyl-2,2’ |
Yoshihira et al., 1970.
|
|
8. |
D. tricolor |
Isodiospyrin Diosquinone |
|
|
9. |
D. canaculata De Wild |
Napththoquinone Coumarin Ismailin Canaculation |
Jeffreys et al., 1985. |
|
10. |
D. mollis |
Tetra hydroxy dimethyl-2, 2’ Binaphthyl |
Loder et al., 1957. |
|
11. |
D. usambarensis Root bark
Stem bark |
7-methyljuglone, Mamagakinone, Isodiospyrin, Diosindigo A 7-methyluglone Diosindigo B Diosindigo A 7-methyljuglone |
Marston et al., 1984.
Mohammad Rafiulla Khar et al., 1989. |
|
12. |
D. leucomelas Leaves |
Betulin Betulinic acid Ursolic acid |
Maria del Garmen Recio et al., 1975.
|
|
13. |
D. chloroxlon Wood |
7-methyljuglone Diospyrin Isodiospyrin Xylospyrin 2-methyl-3, 6-dihdroxy-4, 5 Dimethoxy haphthalenes 2-methyl-3, 4, 5, 6-tetra methoxy-napthalene |
Sidhu et al., 1971. |
The steam – volatile constituents of D. blancoi A.DC has been studied and 24 components of the oil have been identified by RI, IR and MS spectra. The major components of the a–farnesene (Collins et al., 1976).
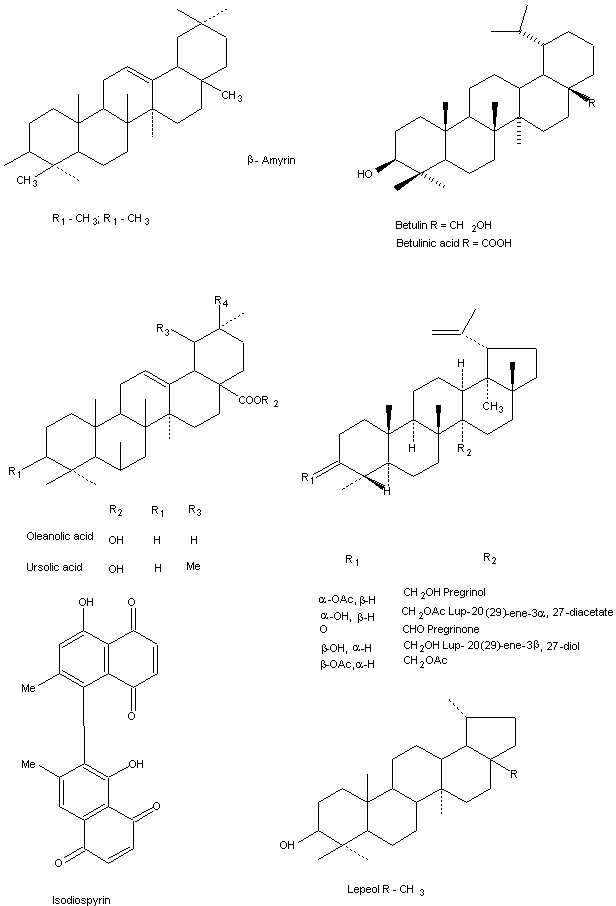
Chemical constituents of Diospyros species
Different classes of compounds have been isolated from different species. They are as follows.
The main components isolated from the Diospyros species are triterpenes and their steroids compounds.
Dichloromethane extract of D. leucomelos Poir leaves isolated three triterpenes betulin, betulinic acid and ursolic acid were identified by 1H – and 13C-NMR spectra studies (Chopra et al., 1956).
The chemical composition of the root of D. lotus (L.) was investigated by Yoshihira et al., 1970, the chloroform extract separated in four napthoquinones, 7-methyljugulone, 150 diospyrin, and quinines besides the three tri-terpenoids, taraxerol, betulinic acid and oxallobetulin.
|
|
|
|
|
A new triterpene was isolated from the fruits of D. peregrina and its structure elucidated as lup 20(20) – en-3a, 27 diol on the basis of spectral analysis (Jeffreys et al., 1985). Maridass, 1999 analyzed the chemical composition by the fruit oil of D. malabarica Desr. by capillary GC and GC/MS studies. More than 35 constituents were isolated of which 29 were identified. The main constituents of trans methyl isoeugenol (31.86%), b-bisabolene (25.91).
Biological activities of Diospyros species
Fifty percent of extracts of D. peregrina minimum tolerated dose of significant activities of antiprotozoal, antiviral and hypoglycemic activity are reported (Yoshihira et al., 1967).
Antibacterial activity
Active constituent of Diosquinone was isolated from D. tricolor inhibited against 11 gram-positive bacteria. Among the gram-positive bacteria active of diosquinone was found to be very active (8.19mm) against Staph aures E3+etc., except S. faecalis and B. cereus (Watt et al., 1980).
Anti-inflammatory activity
Betulin, betulic acid and ursolic acid were isolated from D. leucomelas. The three triterpenoids compounds have been found to exert pronounced anti-inflammatory activity against different model of experimental inflammation (Misra et al., 1967).
Cytotoxic activity
Two cytotoxic compounds isodiospyrin and b-amyrin and in active triterpene, olean-12-3-one have been isolated from D. morrisiana (Collins et al., 1976).
References
1. Anon, 1952. The Wealth of India, Raw Materials, CSIR, New Delhi, India. 3, 85.
2. Bhanmik, T., Dey, A.K., Das, P.C., Mukhopadhay, A.K and Chatterjee, A. 1982. Ind. Chem. Soc., 20B, 664.
3. Burkhill, I.H. 1966. A Dictionary of the Economic products of the Malay Peninsula. Ministry of Agriculture and Co.op., Kuala Lumpur, Malaysia.
4. Chauhan, J.S and Kumari, G. 1978. J. Chem. Soc. 55, 1068.
5. Chauhan, J.S., Sarawat, M and Kumari, G. 1979. Planta Med., 35, 373-375.
6. Chauhan, J.S., Saraswat, M and Kumari, G. 1982. Ind. J. Chem. Soc., B., 21B, 169.
7. Chopra, R.N., Nayar, S.L and Chopra, I.C. 1956. Glossary of Indian Medicinal Plants, CSIR, 99.
8. Collins, R.P and Halim, A.F. 1976. Eco. Bot., 30, 713-716.
9. Dhar, M.L., Dhar, M.M., Dhawan, B.N., Mehrotra and Ray, 1968. Indian J. Exp. Biol., 6, 222-247.
10. Dutta, P.K., Dutta, N.L and Chakravarthi, R.N. 1972. Phytochemistry, 11, 1180-1181.
11. Heynauer, R. 1966. Chemotaxonomy of plants, Birkhauser, Basel. 4, 45.
12. Jeffreys, J.A.D., Zakaria, M.B., Waterman, P.G and Zhong, S.M. 1985. Tetrahedron Lett. 24, 1085.
13. Kirtikar, K.R and Basu, B.D. 1935. Indian Medicinal Plants, II, 1502.
14. Keay, R.W.J., Onochie, E and Standfield, D.D. 1964. Nigerian Trees. 1, 62, FRIN, Ibadan.
15. Kuroanagi, M., Yoshihira, K and Natori, 1971. Chem. Pharm. Bull., 19, 2308.
16. Alake, L.B. 1994. Planta Med., 60, 477.
17. Lee, T.J., Shih, T.S., Liu, Y.M and Chen, F.C. 1984. Formosan Sci., 38, 147.
18. Loder, J.W., Mongolsuk, S., Robertson, A and Whalley, W.B. 1957. J. Chem. Soc., 2233.
19. Mariadel Carmen Recio, Rosa Maria Giner, Manez, S., Gueho, J., Julien, H.R., Hostettmann, K and Rio, S.J.L. 1995. Planta Med. 61, 9-12.
20. Maridass, M. 1999. Essential oils of an ethnomedicine Diospyros malabarica Fruits, M.Sc Thesis, Sri Paramakalyani Centre for Environmental Sci. Alwarkurichi, India.
21. Marston, A., Msonthi, J.D and Hostettman, K. 1984. Planta Med., 279.
22. Misra, G and Mitra, C.R. 1967. Phytochemistry, 6, 1309.
23. Misra, G and Mitra, C.R. 1968. Phytochemistry, 7, 501.
24. Misra, P.S., Misra, G., Nigam, S.K and Mitra, C.R. 1971. Phytochemistry, 10, 904-905.
25. Khan, M.R., Kishimba, M.A and Harry Locksley, 1989. Planta Medica., 581.
26. Muhamad Bin Zakaria, 1989. Planta Med., 55, 204-205.
27. Neeru Jain and Rajnath Yadav, 1994. Phytochemistry, (35)4, 1070-1072.
28. Okogun, J.I., Enenishi, U.V and Ehong, D.E.U. 1978. Tetrahedron, 34, 1221.
29. Ramachandra Row, L., Sanakra Rao, C and Sundara Ramaiah, T. 1969. Indian J. Chem., 7, 204-206.
30. Sidhu, G.S and Prasad, 1971. Indian J. Chem, 9, 767-769.
31. Sidhu, G.S., Sankaran, A.V.B and Mahmood Ali, S. 1968. Indian J. Chem., 6, 681-691.
32. Sturm, V.G and Zilliken, 1969. Planta Med., 21, 61-66.
33. Sundaramaiah, T., Ramraj, S.K., Rao, K.L and Vimalabai, 1976. J. Ind. Chem. Soc., 53, 638.
34. Watt, G. 1980. The Dictionary of Economic Products of India, 3, 141.
35. Wu, S.C., Yang, Y.H., Hsu, K.K and Chen, F.C. 1972. Technical Bull. Exp. Forest, NTU (Taipei), 97, 11.
36. Xiu-Zhen Yan, Yao-Haur Kuo, Tsong-Jyh Lee, To-Shii Shih, Chung-Hsiung Ghen, Donald, R., Mephail, Andrew, T., McPhail, Kuo-Hsium Lee and Andrew, 1989. Phytochemistry, 28, 5, 1541-1543.
37. Yoshihira, K., Michiko Tezuka and Natori, 1970. Tetrahedron Letter, 1, 7-10.
38. Yoshihira, K., Natora, S and Pandia Kanchanapee, 1967. Tetrahedron Letters, 48, 4857-4860.
39. Yoshihira, K., Tezuka, M and Natori, S. 1970. Tetrahedron Letters, 1, 7.
40. Zakaria, M.B., Jeffreys, J.A.D., Waterman, P.G., Zhong, S.M. 1984. Phytochemistry, 23, 1481.