|
Ethnobotanical Leaflets 12: 577-85. 2008.
Callus Induction and Plant Regeneration of Vigna mungo (L.) Hepper via Half Seed Explant
*Harisaranraj, R., S. Saravana Babu and Suresh, K.
Department of Plant Biology and Plant Biotechnology Chikkaiah Naicker College, Erode. (T.N.) INDIA
Issued 11 August 2008
Abstract
The present study optimized the regeneration protocol by using half seed explant in Vigna mungo (L) Hepper organogenesis. Half seed explants were inoculated onto B5 medium supplemented with kinetin (4.7 M to 23.5 M), 6- benzyladenine (4.4 M to 22.2 M), naphthaleneacetic acid (5.4 M to 27.0 M), indolebutyric acid (4.9 M to 24.5 M) and 2,4- dichlorophenoxyacetic acid (4.5 M to 22.5 M). Callus initiation was observed in all media evaluated and the highest cell proliferation was obtained from explants cultivated in the presence of 13.3 M BAP and 13.5 M 2,4-D. Shoot induction was obtained from callus induced on 13.3 M BAP and 13.5 2,4-D at 6 weeks after transferring the callus to a B5 medium supplemented with 13.3 M BAP. Roots were induced from shoots on B5 media with indolebutyric acid (IBA, 14.7 M) and then regenerated plants were hardened and acclimated in greenhouse conditions.
Key Words: Vigna mungo, callus induction, half seed, rooting, shoot regeneration.
Introduction
Vigna mungo (L) Hepper (Black gram) is considered to have been domesticated in India from its wild ancestral form V.mungo var.silvestris (Lukoki, Marechal & Otoul, 1980). Center of genetic diversity is found in India (Zeven and de Wet. 1982). Natural distribution of V.mungo var.silvestris ranges from India to Myanmar (Tateishi. 1996). Vigna mungo (L) Hepper (Black Gram or Urd Bean) is one of the most widely used pulse crop in India. It is a highly prized pulse, very rich in phosphoric acid. It is cultivated as fallow crop after rice cultivation in India. It is grown in various agro-ecological conditions and cropping systems with diverse agricultural practices (Sanjeev Gupta et al). The improvement and optimization of Vigna mungo characteristics, such as increasing resistance to pests, lowering allergenic protein levels in seeds, drought and salt tolerance is therefore desirable.
The crop improvement was done by breeding methods in early days. However, breeding is difficult due to the fact that Vigna mungo is self-pollinating crop and the genetic variation among the Black gram varieties is narrow. The regeneration system used to generate genetically modified plants was somatic embryogenesis from immature seeds (Christou et al., 1989) or organogenesis from cotyledonary nodes (Finer and McMullen, 1991; Shan et al., 2005). In Vigna mungo, genetic modification is also based on organogenesis from the cotyledonary nodes, a high efficient regeneration system was obtained by sub culturing nodes on Thidiazuron (TDZ) supplemented medium (Tzitzikas et al., 2004). Medium components with quantity of plant growth regulator influence the regeneration of plants (Shan et al., 2005). Callus regeneration is advantageous over direct regeneration for transformation since effective selection of transgenic cells can be achieved. However, the efforts made to regenerate plants from callus have yielded poor results since plants could not be regenerated from any type of Vigna mungo callus (Hu and Wang, 1999). In attaining this goal, we owe much to transformation techniques for producing new breeding materials that would not be available in the germplasm among cross-compatible species. A number of successful regeneration protocols have been developed, especially with a view to facilitate genetic transformation.
The earlier reports focused on the cotyledonary node since the morphogenetic potentiality is confined to that region (Hu and Wang, 1999). Eventhough plants have been successfully transformed by using cotyledonary nodal explants, the major problem of chimerism still persists (Meurer et al., 1998). This is primarily due to pre-existing meristematic shoot buds, which continue to grow effectively on a medium containing a selectable agent like antibiotic or herbicide. Christou et al. (1988) could effectively select transgenic calli after bombarding protoplasts but they failed to produce transgenic plants. The callus induction and plant egeneration of Indian soybean (Glycine max (L.) Merr. cv. CO3) via half seed explant culturewas carried out by (B. D. Ranjithakumari et al). In this present investigation was to standardize the optimum protocol for callus and shoot regeneration from half seed explant of the Vigna mungo.
Materials and methods
Matured Vigna mungo seeds were utilized in the efficient production of in vitro Vigna mungo plant production experiments. Vigna mungo seeds were washed under continuous flashing of running tap water for 30 min and then treated with a solution of the Tween 20 (5% v/v) for 10 min and finally surface sterilized with HgCl2 (0.1% w/v) for 10 min. Lastly, the seeds were washed three times with autoclaved distilled water to remove any trace of
Half seed explants preparation
Disinfected seeds were soaked in sterile distilled water for about 4 h and a longitudinal cut along the hilum was made to separate the cotyledons, and the seed coat was removed. The embryonic axis found at the junctions of the hypocotyl and cotyledon was excised to obtain the half- seed explants.
Callus induction
Initial explants consisted of half seed (ie, the part of the explant from where the embryonic axis was removed) was cultured in flate side up in the medium with the base of the explant was embedded condition. The callus initiation medium contained B5 salts and vitamins (Gamborg et al., 1968), 30 g l-1 sucrose. The pH was adjusted to 5.8 and solidified with 0.7% agar before autoclaving at 121C for 20 min. The effect of growth regulators was tested using KIN (kinetin), BAP (6-benzyladenine), NAA (naphthaleneacetic acid), IBA (indolebutyric acid), 2,4D (2,4dichlorophenoxyacetic acid) as follows: KIN (4.7 M to 23.5 M), BAP (4.4 M to 22.2 M) and NAA (5.4 M to 27.0 M) or IBA (4.9 M to 24.5 M) or 2,4D (4.5 M to 22.5) in combination with BAP (4.4 M to 22.2 M). Medium without plant growth regulators was used as a control. Cultures were maintained under light 16h or dark 8 h conditions at 262C. 50 half seed explants were inoculated per treatment and repeated 3 times and the frequency of callus formation was determined 4 weeks after culture initiation.
Shoot induction and multiplication
To induce and proliferate shoots, the calli were transferred to shoot induction medium containing B5 salts, B5 vitamins, 30 g l-1 sucrose, pH 5.8, 0.7% agar and fortified with BAP (4.4 M to 22.2 M). Calli were kept under the same light/ darkness conditions from the callus initiation for 4 weeks. Depending on the treatment, the number of shoots per treatment was recorded at 6 and 10 weeks after transferring the calli to shoot induction medium.
Rooting and Hardening of plantlets
For root induction, 14.7 M IBA was tested. Elongated shoots (1.5 to 2.5 cm long) were isolated and transferred to B5 medium, supplemented with B5 vitamins, 30 g l-1 sucrose, pH 5.8, 0.7% agar and 14.7 M IBA. After 3 weeks, rooted plantlets were rinsed with water to wash off the agar medium and then, transplanted to soil containing cups for hardening. The hardened plants were maintained at 242C with 18 h photoperiod (140 moles s-1) for 2 weeks then, transferred to the greenhouse.
Statistical analysis
The various concentrations of plant growth regulators (KIN, BAP, BAP+ NAA, BAP+ IBA and BAP+ 2, 4 D) were tested in callus induction and plant regenerations. Frequency of callus and shoot initiation, number of shoots were analyzed by SPSS software, in which statistical significance was determined at the 0.05 probability level.
Results and discussion
Half seed explant is an efficient source for the Vigna mungo callus initiation and shoot regeneration. The half seed derived cotyledonary nodal callus could serve as an ideal starting material for developing an efficient Vigna mungo transformation system. (Sairam et al., 2003).
Callus initiation
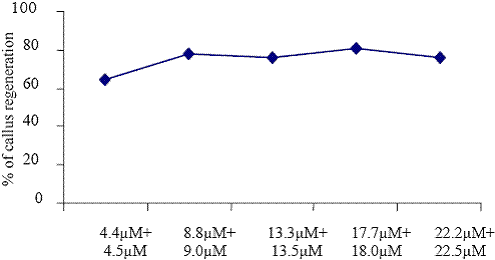
The regeneration of callus induction and plant regeneration were influenced by different kinds of plant growth regulator (KIN, BAP, NAA, IBA and 2,4D). Callus was induced in all media tested and significant differences were observed in the induction frequency between different plant growth regulators (Fig. 1 to 5). Among the various plant growth regulators, the highest frequency of callus induction was observed to be when the half seed explants were cultured in the presence of BAP (13.3 M) + 2,4D (13.5 M) (Fig. 5). These observations indicate that the morphogenetic potential is confined to only the cotyledonary nodal cells as reported by Hu and Wang (1999).
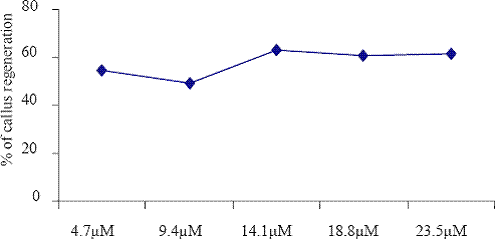
Fig. 1. The effect of different concentrations of KIN on callus regenerations.
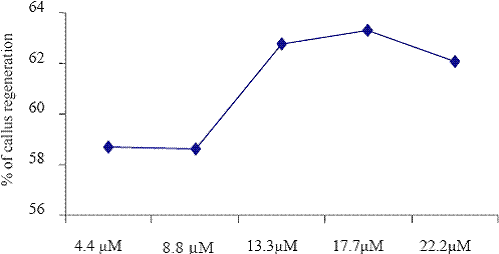
Fig. 2. The effect of different concentrations of BAP on callus regenerations.
The low frequency of callus induction was observed in KIN (14.1 M) exposed explants cultured on the B5 medium. The explants grown in B5 medium supplemented with BAP (4.4 M to 22.2 M) and different auxin combination such as NAA (5.4 M to 27.0 M), IBA (4.9 M to 24.5 M) and 2,4D (4.5 M to 22.5) were given the better yield than that of KIN (4.7 M to 23.5 M). Barwale et al. (1986a) reported that the organogenic callus cultures were obtained from immature soybean embryos grown on the medium with a high 6-benzylaminopurine (BAP) concentration (13.3 M) and 0.2 M NAA.
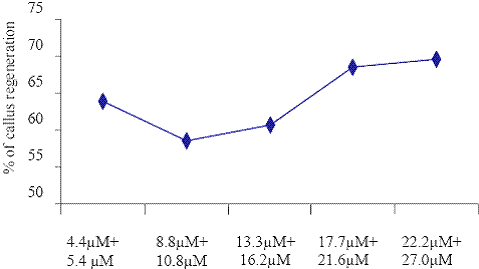
Concentrations of BAP+ NAA
Fig. 3. The effect of different concentrations of BAP + NAA on callus regenerations.
Plantlet regeneration
Half seed explant derived calli were cultured on the medium fortified with various concentrations of BAP supplemented B5 medium (SI medium). In Vigna mungo, BAP was found to be the most efficient in shoot formation when excised parts of mature and immature cotyledones were used (Sairam et al., 2003). 13.3 M BAP + 13.5 M 2, 4 D treated callus produced the maximum number of shoots than other concentrations of BAP alone (Table 1). The marked difference observed between the average number of shoots obtained from callus induced from different concentrations and combinations of BAP with 2, 4 - D could be related to the media used for callus induction and multiplication. Callus induced on 13.3 M BAP + 13.5 M 2, 4 D formed the highest number of shoots after 6 weeks in the presence of BAP treated SI medium (Table 1). The former calluses were completely covered with shoot buds were easily disintegrated during manipulation. However, the shoots could be excised from their surface. The calli could be kept for six months of subcultures producing an average of 6 shoots per callus in every month. The procedures that have been established for shoot induction and multiplication of Glycine species have reported an average from 2 to 5.5 shoots, depending on the explant used (Mederos et al., 1997; Moura, 1998). These calli could be maintained in a long term process of shoot induction. The maximum number of shoots was regenerated to 13.3 M BAP supplemented with SI medium. It is in contrast with BAP, alone or in combination with NAA, has previously been reported as being efficient in promoting shoot differentiation in several species (Blakesley and Constantine, 1992).
Table 1
Use of 5.0 M BAP significantly increased the regeneration frequency of shoots from the Vigna mungo cotyledonary node. Wright et al. (1986) and Hinchee et al. (1988) also obtained shoot regeneration on cotyledonary explants at 5.0 M BAP. Use of 7.5 M BAP [as routinely used in cotyledonary node protocol; Olholf et al., (2003); Paz et al. (2004)] gave the lowest regeneration rate when applied to the half- seed system. However, for H. canariense and H. foliosum, the best results for shoot induction were obtained directly from apical or axillary buds cultured in media supplemented with BAP and NAA (Mederos, 1991; Moura, 1998).
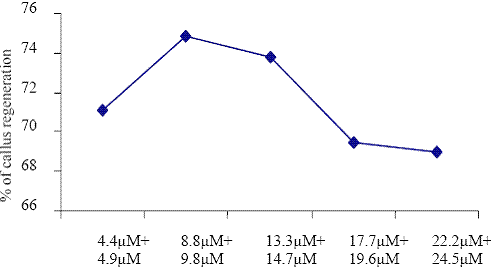
Concentrations of BAP+IBA
Fig. 4. The effect of different concentrations of BAP + IBA on callus regenerations.
Barwale et al. (1986b) reported that, the best multiple shoot induction was observed in B5 medium supplemented with 1.0 or 5.0 M BAP in several Vigna mungo genotypes. Paz et al. (2006) suggested that culture condition optimized in the cotyledonary node system were not immediately applicable to the half-seed protocol and by improving an optimum hormonal environment to the explant in vitro, the ability of transformed cells to regenerate into plants may be improved. In Vigna mungo, elongated shoots obtained from callus were rooted in B5 medium with 14.7 M IBA. The IBA was an efficient auxin to produce the shoots (Paz et al., 2006).
Concentrations of BAP+2, 4 D
Fig. 5. The effect of different concentrations of BAP + 2,4 D on callus regenerations.
Similar results have been described for some species of Hypericum (Mederos, 1991; Moura, 1998). The presence of IBA on the rooting media seemed to have an effect on the number of roots induced per shoot. Three weeks were enough to develop strong and healthy plantlets with good root systems, ready to be transplanted and acclimated. The plantlets developed in vitro were transferred to sterile soil and maintained in controlled conditions in a growth chamber for one week. In this period of time, the pots were watered regularly. After 2 week, the plants were taken to the greenhouse. In conclusion, using plant growth regulators, the efficient callus mediated regeneration from half seed explant of Vigna mungo has been standardized. The half seed callus could serve as an ideal starting material for developing an efficient Vigna mungo transformation system.
Acknowledgment
Authors are gratefully thankful to Prof. K. Sekar, Lecturer (SG), A.A Government Arts College, Namakkal, Tamilnadu, India for constant encouragement and timely help during the present work. We are also greatful to the Directors, BIIOGENEIC Institute of Biotechnology, Namakkal, Tamilnadu, India, for providing laboratory facilities.
References
|