|
Ethnobotanical Leaflets 12: 320-336. 2008.
Toxicity of Gynandropsis pentaphylla DC Extracts Against Microbials and Its Phytochemical Profile
J. Francis Borgio1, Pravin K. Thorat2 and Archana D. Lonkar2
1Genecity Laboratories Pvt. Ltd., 128 / 1B, First Floor, Chhatrapati House, Paud Road, Kothrud, Pune 411038, India. E.mail: [email protected] 2 Maa Saraswati Medi Education Society, College of Paramedical Sciences, Hardikar Hospital Campus, Ganesh Khind Road, Pune 411 001, India. Email: [email protected]
Issued 28 May 2008
Abstract Fresh leaves, roots, stems, seeds and seed pods of Gynandropsis pentaphylla DC were used in extractions with benzene and ether. Fresh extracts were tested against 6 bacteria and 4 fungi using the well diffusion method. Extracts of roots, stems, leaves, seeds and seed pods were screened phytochemically for the presence of secondary metabolites such as tannin, alkaloid, flavones, sugar, phenolic group, essential oil, amino acids and saponin. All the phytochemicals were observed in all the plant part extracts using both ether and benzene. Among the bacterial cultures, Agrobacterium tumefaciens (NCIM 2145) was highly susceptible (28.00.3 mm) to both ether and benzene extracts of the leaves of G. pentaphylla. Also A. tumefaciens showed the highest average zone of inhibition for all extracts. Among the fungi, Penicillium notatum (NCIM 747) was highly susceptible (25.00.0 mm) to the benzene extracts of G. pentaphylla leaves. The extracts of leaves showed highest activity among all the plant parts. Highest activity index was observed for all extracts against the fungus P. notatum.
Key Words: Toxicity, Antifungal assay, Bacteria, Fungi, Gynandropsis pentaphylla, Phytochemical study.
Introduction The leaves and seeds of cats whiskers (Gynandropsis pentaphylla DC.) are used in indigenous medicine in many countries (Chweya and Mnzava, 1997; Ajaiyeoba et al., 1998). Cats whiskers grow as a weed in most tropical countries. In India the common names include hurhur and karaila (Chweya and Mnzava, 1997). Gynandropsis pentaphylla (synonyms: Gynandropsis gynandra L. (Briq.) and Cleome gynandra L. (Briq) (Chweya and Mnzava, 1997) belong to the plant family Capparidaceae. Gynandropsis pentaphylla DC. It is an herb indigenous to the tropical and sub tropical regions. The herb is edible and grows to 60cm high (Dalziel, 1937; Burkhill, 1985; Irvine, 1961; Adjounahun and Ake Assi, 1972). Hurhur has been used for several years in Indian traditional medical practices. G. pentaphylla leaves with a high percentage of vitamin C is taken as a pot herb in soups, fresh or dried (Chweya and Mnzava, 1997). The leaves are used as disinfectants. Inhalation of the leaves also relieves headaches; leaf juice and oil, for earache and eye wash (Chweya and Mnzava, 1997). Seeds have been reputed to have antihelmintic properties and oil is used as fish poison (Walker and Sillans, 1953; Walker, 1953; Chweya and Mnzava, 1997). Sterols are also found in these plants; with lupeol, campesterol and epi-lupeol having been isolated from this palnt (Kondagbo et al.,1973; Lakishimi and Chanhan, 1977). In previous studies, the anthelmintic and antimicrobial properties of Capparidaceae plants have been reported (Ajaiyeoba and Okogun, 1996; Ajaiyeoba, 2000) from different countries and in continuation of these objectives on this plant family, the phytochemical, antibacterial and antifungal properties of Gynandropsis pentaphylla are presented in the current study for the Indian G. pentaphylla. G. pentaphylla plants have been observed to have insecticidal, antifeedant and repellent characteristics (Akhtar, 1990; Malonza et al., 1992; Pipithsangchan, 1993; Chweya and Mnzava, 1997). The leaves have anti-tick properties. They also have repellent and acaricidal properties for larvae, nymphs and adult Rhipicephalus appendiculatus and Amblyomma variegatum ticks. Ticks may not be found for a distance of 2-5 m from the plant (Chweya and Mnzava, 1997). The ethanol extract is toxic to insect pests, such as the painted bug (Bagrada cruciferarum Kirk) and the diamond back moth (Plutella xylostella L.) of cruciferous vegetables. The volatile oils permanently repel the diamond back moth larvae from treated cabbage leaves. The plant has an anti-feedant action against the tobacco caterpillar (Spodoptera litura F.). The extract from the mature seeds is toxic to brinjal aphid (Aphis gossypii Glov.), and the larvae of Helicoverpa armigera (Hubner). The seeds contain phenolic compounds, which are natural products (Jain and Gupta, 1985; Chweya and Mnzava, 1997). Lipids from seeds could be used in soap manufacture (Gupta and Chakravarty, 1957; Chweya and Mnzava, 1997). The biocontrol agent, Metarhizium anisopliae (Metsch.) Sorokin (Deuteromycotina: Hyphomycetes) is an extensively studied cosmopolitan filamentous fungus, which is a key regulatory organism of insect populations (Borgio and Sahayaraj, 2007). No reports were available on the activity of G. pentaphylla against this entomopathogenic fungus and also against A. chrococcum, L. acidophilus, A. tumefaciens, P. notatum. With these views in mind, the present study was designed to test the toxicity of leaves, roots, stems, seeds and seed pods extracts of indigenous G. pentaphylla against the following six bacterial and four fungal species: Azatobacter chrococcum, Bacillus subtilis, Lactobacillus acidophilus, Agrobacterium tumefaciens, Escherichia coli, Staphylococcus aureus, Aspergillus niger, Aspergillus flavus, M. anisopliae, Penicillium notatum.
Materials and methods Preparation of plant extract Gynandropsis pentaphylla was collected from Rajgurunagar area of Pune, Maharashtra, India in March 2008. About 200g of G. pentaphylla leaves were ground in mortar and pestle with 10ml of benzene. Resulting paste collected in a conical flask and allowed to mix properly by placing it in a shaker at 200 rpm for 24 hr. Then the paste was filtered through double layered muslin cloth, the filtrate of leaves in water was used for the further studies. Similar protocol was adapted to prepare leaves extract using ether. The same procedure was used to prepare the extract from other plant parts like roots, stems, seeds and seed pods. All the extracts were stored at 4oC for further analysis.
Phytochemical analysis Qualitative phytochemical evaluation was carried out to test the presence of alkaloids, flavones, sugar, phenolic group, saponin, amino acid and essential oil in the extracts samples using modified method of Brindha et al. (1981).
Tannin: A test solution (500l) was made with distilled water (500l), to which 0.01g lead acetate was added. The development of a white turbidity in the precipitate represented the presence of tannin.
Alkaloids: A test solution (500l) was made with 2N HCl (500l). The aqueous layer was decanted. To the lower layer 2 drops of Mayers reagent was added. Development of a white turbidity in the precipitate represented the presence of alkaloids
Flavones: The test solution (500l) was mixed with 100l of alcohol, 0.02g of paradimethyl amine benzaldehyde and two drops of conc. HCl. Development of red or pink color indicated the presence of flavones
Sugar: The test solution (500l) was made in a clean test tube, to which 0.01g of anthrone and 3 drops of conc. H2SO4 were added. The solution was heated for 1 to 2 minutes. Change of green to purple color was noted to detect the presence of sugar in the sample.
Phenolic group: An alcoholic plant extract (500l) was prepared in a test tube. Two drops of 1M ferric chloride were added. Appearance of intense color indicated the presence of phenolic groups.
Saponin: A test solution (500l) with distilled water (2 drops) was prepared in a test tube. The development of a foamy lather indicated the presence of saponin.
Amino acid: A test solution (500l) made with two drops of 1% ninhydrine in alcohol was prepared in a test tube. Blue or violet color development indicated the presence of amino acid.
Essential oil: A test solution (500l) made with two drops of 1M alcoholic K2Cr2O7 and 3 drops of phenoptheline was prepared in a clean test tube. Soap formation indicated the presence of essential oil.
Assay of Toxicity Levels Chosen microorganism used Pure cultures of Bacillus subtilis NCIM 2010, Lactobacillus acidophilus NCIM 2660, Agrobacterium tumefaciens NCIM 2145, Escherichia coli NCIM 2064, Staphylococcus aureus NCIM 2120, Aspergillus niger NCIM 501, Aspergillus flavus NCIM 650, Metarhizium anisopliae NCIM 1311, Penicillium notatum NCIM 747 were obtained from the National Collection of Industrial Microorganism (NCIM), National Chemical Laboratory, Pune, India. Azatobacterchrococcum Gcity 01 was obtained from Genecity Laboratories Pvt. Ltd, Pune.
Media used Luria agar (LA) (for B. subtilis, E. coli and S. aureus) potato dextrose agar (PDA) (for A. niger, A. flavus, M. anisopliae and P. notatum) (HiMedia, Mumbai, India), YEB medium (A. tumefaciens), Azotobacter ATCC medium (A. chrococcum) and MRS medium (L. acidophilus) were used for the bacterial and fungal bio assays.
Antimicrobial agents Cloroamphenicol (10mg/ml) and fluconozole (10mg/ml) (HiMedia, Mumbai, India) were used as standard antibacterial and antifungal agent respectively. They were included in the current study as standard reference drugs.
Determination of the Toxicity Levels of G. pentaphylla Sterile cotton swabs were dipped in a 24 hour-old S. aureus culture. The entire agar surface of LA plate was seeded first in horizontal direction and then in vertical direction to ensure the even distribution of organism over the agar surface using the above swab. The seeded agar surface was allowed to dry for five minutes. Tip of well cutter was sterilized by heating on the blue flame of Bunsen burner and used for the well preparation in the seeded LA plates. Three wells were prepared in each LA plate. As soon as the wells were prepared all plant part extracts (10l) were poured in each well separately using sterile micro-tips. The same procedure was followed for A. tumefaciens, B. subtilis, E. coli, A. chrococcum L. acidophilus A. niger, A. flavus, M. anisopliae and P. notatum in their respective plates using 24 and 48 hours of broth culture for bacteria and fungi respectively. Antimicrobial agents [Cloroamphenicol (10mg/ml) and fluconozole (10mg/ml)] were poured as positive control and respective solvents were used as negative control. LA, azotobacter media, agrobacterium media and MRS media plates were incubated at 372oC for 24 hrs and PDA plates incubated at 272oC for 72 hrs. After incubation the zone of inhibition was measured using HiAntibiotic Zone Scale (HiMedia, Mumbai, India).
Activity Index The zone of inhibition in extract and the standard antimicrobial agents were used to calculate the activity index. Zone of inhibition by extract AI = ________________________________________________________________ Zone of inhibition by standard antimicrobial agents
Proportion index Number of positive results obtained for water, methanol and acetone extract of one plant part was against all the microbials and total number tests carried out were used to evaluate the proportion index. No. of positive results Proportion index (%) = _____________________________ X 100 Total number tests
Statistical analysis The zone of inhibition was subjected to cluster analysis to reveal the relativeness of the activity among the human pathogens using STATISTICA/w 5.0. software. A correlation analysis between the pathogens and also different extracts were performed using STATISTICA/w 5.0. software.
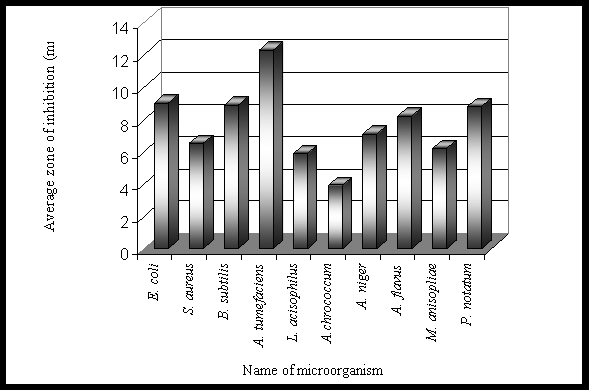
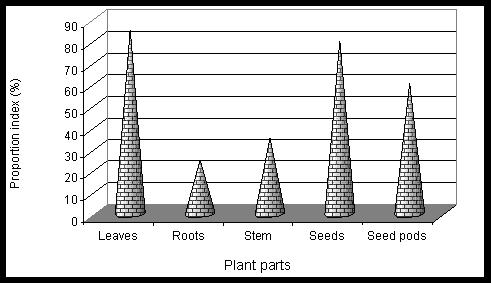
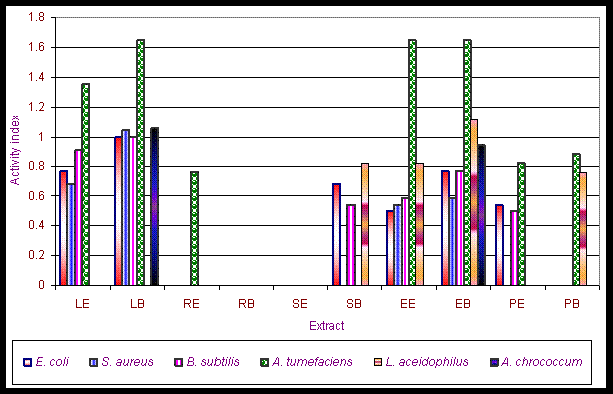
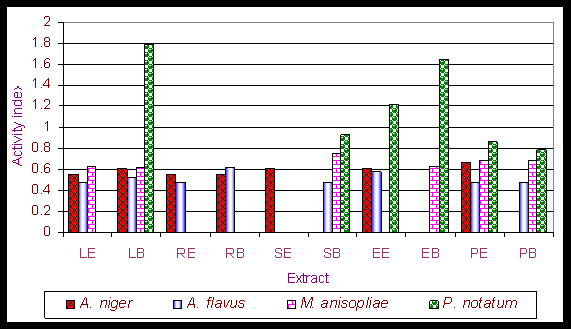
Results and Discussion The phytochemical screening of Gynandropsis pentaphylla revealed the secondary metabolites which are of medicinal interest as presented in table 1. G. pentaphylla is rich in tannin, sugar, essential oils, amino acid and phenolic group. However, in G. pentaphylla seeds extract (both benzene and ether solvents) contained tannins, alkaloids, flavones, sugar, phenolic group, saponin, amino acid and essential oil in plenty of amounts (table 1). Flavons and saponins were comparatively less in this species extracted using both benzene and ether solvents. The variance in the quantitative composition of the secondary metabolite establishes the fact that different plant parts are not likely to have the same medicinal potential. Leaf contained all the secondary metabolites screened. These secondary metabolites are known to exhibit medicinal activity as well as physiological activity (Ross, 2005). Presence of alkaloids and reducing sugars in leaves and stems were reported by Ajaiyeoba et al. (1998). In the present study we also observed the same results in stem and leaves. Apart from both leaves and stems we additionally observed the presence of alkaloids and reducing sugars in seeds, roots and seed pods extracts (ether and benzene) of G. pentaphylla. This is not suprising for plants of the Capparidaceae family (Kjaer et al., 1973; Lakishimi and Chanhan, 1977; Ajaiyeoba et al., 1998.). The results of the antimicrobial activity presented in table 2 shows that both benzene and ether extracts exhibited appreciable antimicrobial properties, inhibiting the growth of all microorganisms. While both benzene and ether extracts of roots not inhibit the growth of Azatobacterchrococcum, Bacillus subtilis, Lactobacillus acidophilus, Agrobacterium tumefaciens, Escherichia coli, Staphylococcus aureus, M. anisopliae, Penicillium notatum. Two clinical strains of human pathogenic fungi like A. niger and A. flavus and one entomopathogenic fungi, Metarhizium anisopliae and also penicillin producer P. notatum were used to find out the antimycotic activity of G. pentaphylla. P. notatum was the most sensitive; this was closely followed by A. flavus, A. niger and M. anisopliae. Average zone of inhibition of activities of G. pentaphylla extracts against microorganism is presented in Figure 1. The Average zone of inhibition was highest in Agrobacterium tumefaciens for both the extracts of all parts of G. pentaphylla. Proportion index of antimicrobial activities of G. pentaphylla extracts shows the highest activity in leaves extract as presented in Figure 2. The relationship between the sensitivity patterns of different microbials against both ether and benzene extracts used for the antimicrobial assay had been studied using STATISTICA/w 5.0. software (Table 3). A high correlation coefficient (near 1 or -1) means a good relationship between two variables and its value around zero means no relationship between them at a significant level of p < 0.05. More precisely, it can be said that parameters showing r > 0.7 are considered to be strongly correlated whereas r between 0.5 and 0.7 shows moderate correlation. In the present study, the resultant matrix reveals strong positive correlation of different bacteria (Table 3) used. (r > 0.7). A data matrix of ten microorganisms and 13 variables (different extracts with two solvents) were used for this cluster analysis, the results are presented in Figure 3. The microbes were separated into two main groups I and II based on their sensitivity: the bacteria, A. tumefaceiens with the highest resistant was clustered alone in group I and all the remaining organism with moderate and low resistant were grouped into II (Figure 3). In the group II, only one fungi, P. notatum was sub clustered into IIB, which is the second resistant organism (Figure 3). G. pentaphylla extracts were more active against fungi than that of bacteria as presented in Figure 4 and Figure 5. From the result of the antibacterial studies as shown in Table 2, all the extracts exhibited appreciable antibacterial properties, inhibiting the growth of all the bacteria and fungi. The same results were repotted by Ajaiyeoba et al. (1998). (Rahman and Choudhary, 1998). In most instances, activities were lesser than the standard therapeutic agent, chloramphenicol as presented in Table 2. The current study was correlated with the previous report (Ajaiyeoba et al., 1998). However, in the current investigation we havent observed any resistant bacteria to chloramphenicol. In the antimycotic assay, P. notatum was highly susceptible (25.00.0 mm) to the benzene extracts of G. pentaphylla leaves. Highest activity index was observed generally against P. notatum. M. anisopliae was moderately sensitive to G. pentaphylla extracts. M. anisopliae (Borgio and Sahayaraj, 2007) and G. pentaphylla (Chweya and Mnzava, 1997; Tvedten, 2007) are active pest control agents, since both are incompatible to each other, it is difficult to use the both pest control agents simultaneously in integrated pest management (IPM). Farmer friendly A. chrococcm and A. tumefecians are highly sensitive to G. pentaphylla extracts. Further studies are needed to evaluate the relationship between these bacteria to G. pentaphylla extracts in field condition. Conclusively, all extracts have displayed antimicrobial activities from present studies. The leaves and seeds of cats whiskers are used in indigenous medicine in many countries (Chweya and Mnzava, 1997). This has further confirmed the use of this plants in Indian ethnopharmacology for treatment of bronchitis, boils, earache, eye wash, disinfectant and nasal congestion, analgesic, headaches, epileptic fits, facilitate childbirth in pregnant women, stomach-ache, constipation, conjunctivitis, thread-worm infection, chest pains, arthritis, inflammation, neuralgia, rheumatism, localized pains, pus, anaemia, uterine complaints, malaria, pneumonia, head lice and reduce coughing (Burkhill, 1985 Ainsle, 1937; Kerharo and Bouquet,1950; Irvine, 1961; Chweya and Mnzava, 1997; Joy et al., 1998; Ajaiyeoba et al., 1998). There is need for the development of new antibiotics due to acquired resistance, more importantly from natural sources as this delays resistance. G. pentaphylla used for the study provide good opportunities for drug development in this area. Further studies are needed to find out the exact compound responsible for the antimicrobial activity using thin layer chromatography, column chromatography and HPLC. Further purification and bioactivity is in progress in our laboratory and will be communicated in future.
Acknowledgement Acknowledgements are due to GeneCity Laboratory Pvt. Ltd., Pune for the laboratory facilities. Many thanks to Prof. Mrs. Gandhe, Modern Collage, Pune, for plant identification. PKT and ADL are thankful to Mrs. Damle, Department of Biochemistry, Garware College, Pune for the encouragement. Senior author (JFB) is grateful to Mr. Jagdish Nagdev for encouragement words.
References 1. Adjounahun E. and Ake Assi L. 1972. Plantes Pharmaceutiques de Cote d`Ivoire; 117-118. 2. Ahmed ZF. Hammounda FM. and Seit et Nasr MM. 1972. Naturally occurring glucosinolates with special reference to those of the family Capparidaceae. Planta Medica, 21: 35-60. 3. Ainsle JR. 1937. List of plants used in Native medicine in Nigeria. Oxford University Press, London. 4. Ajaiyeoba EO. and Okogun JI. 1996. Anthelmintic activity of a root extract of Ritchiea capparoides var. longipedicallata. Phytotherapy Research, 10: 436-437. 5. Ajaiyeoba EO. Rahman AU. and Choudhary IM. 1998. Preliminary antifungal and cytotoxicity of extracts of Ritchiea capparoides var. longipedicellata. Journal of Ethnopharmacology, 62: 243-246. 6. Ajaiyeoba, EO. 2000. Phytochemical and antimicrobial studies of Gynandropsis gynandra and Buchholzia coriaceae African Journal of Biomedical Research, (3): 161 - 165. 7. Akhtar MN. 1990. Phytochemical Screening of the Medicinal Plants Opuntia coccinellifera, O. dillenii, O. stricta, Capparis decidua, Cleome viscosa, C. branchycarna, Crateava religiosam and Gynandropsis gynandra of Faislabad suburbs Pakistan, belonging to the Cactaceae and Capparidaceae families. University of Agriculture, Faisalabad, Pakistan, 75 pp. 8. Borgio JF. and Sahayaraj K. 2007. Bioefficacy of the entomopathogenic fungus, Metarhizium anisopliae (Metsch.) Sorokin (Deuteromycotina: Hyphomycetes) on Dysdercus cingulatus (Fb.) eggs. In: Proceedings of the National Seminar on Technology and Management of Bioresearches. Eds. M. Narayann, T. Sethuramalingam, K. Sahayaraj; 2934. 9. Brindha P., Sasikala B. and Purushothman KK. 1981. Pharmacognostic studies on Murugan kizhangu. Bulletin of Medicinal and Ethanobotanic Research ; 3 (1): 84 96 10. Burkhill HM. 1985. The useful plants of W. Tropical Africa, Royal Botanical Gardens, Kew, 318-388. 11. Chweya JC. Mnzava NA. 1997. Cats whiskers. Cleome gynandra L. Promoting the conservation and use of underutilized and neglected crops. 11. Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute, Rome, Italy. 12. Dalziel JM. 1937. The useful plants of Tropical Africa, Crown agents for the colonies, London; 18-22. 13. Gupta AS. and Chakravarty MM. 1957. Studies on the seed for composition of desert plants. Part 1. The component fatty acids of Gynandropsis pentaphylla seed fat. Science and Culture, 23:306-307. 14. Irvine JR. 1961. Woody plants of Ghana. 2nd ed., Oxford University press, London; 51. 15. Jain AC. and Gupta SM. 1985. Minor phenolic components of the seeds of Gynandropsis gynandra. Journal of National Proceedings, 48(2):332-333. 16. Joy PP. Thomas J. Mathew S. and Skaria BP. 1998. Medicinal plants, Kerala Agricultural University, Aromatic and Medicinal Plants Research Station, Kerala, India 17. Kerharo J. and Bouquet J. 1950. Plantes Medicinalis de la Cote dIvoire et Haute Volta, , Paris, p. 289. 18. Kjaer A. Schuster A. Delaveau P. and Kondagbo B. 1973. Glucosinolates in Boscia senegalensis. Phytochemistry; 12: 725. 19. Kondagbo B. Delaveau P. and Adjanohun B. 1973. African Capparidaceae, Boscia senegalensis. Annals of Pharm. Fr; 30: 93-98,. 20. Lakishimi V. and Chanhan JS. 1977. A new pentacyclic triterpinoid from the root bark of Craeteva nurvala. Planta Medica, 32: 214-216. 21. Malonza MM. Dipeolu OO. Amoo AO Malonza MM. and Hassan S.M. 1992. Laboratory and field observations on anti-tick properties of the plant Gynandropsis gynandra (L.) Briq. Veterinary Parasitology, 42(1-2):123-136. 22. Pipithsangchan, S. 1993. Insecticidal activity of selected Thai plants on diamondback moth, Plutella xylostella (L.) (Lepidoptera: Ypnomeutidea). Univ. Philippines, Los Banos, College, Laguna, Phillipines. Monograph. 23. Ross IA. 2005. Medicinal Plants of the World - Volume 3, Chemical Constituents, Traditional and Modern Medicinal Uses, Humana Press Totowa, New Jersey; pp. 631 24. Tvedten S. 2007. The Best Control II, Encyclopedia Integrated Pest Management (IPM) http://www.stephentvedten.com/. 25. Walker AR. and Sillans R. 1953. Les plantes utilis du Gabon., Paul Lechevalier, Paris; pp 117-118. 26. Walker AR. 1953. Usages Pharmaceutiques des plantes spontanus du Gabon. Insitut dEtudes Centra Fracaines; 9: 13-26..
Table 1. Phytochemical screening of Gynandropsis pentaphylla extracts.
+ + + excellent; + + good; + moderately positive; doubtful
Table 2. Antimicrobial activities of G. pentaphylla extracts.
SAA - Standard antimicrobial agent Figure 1. Average zone of inhibition of activities of G. pentaphylla extracts against microorganism.
Figure 2. Proportion index of antimicrobial activities of G. pentaphylla extracts.
Table 3. Correlation matrix of sensitivity of different microorganism by G. pentaphylla extracts.
* Marked correlations are significant at p < .05000
Figure 3. Cluster analysis of sensitivity of 10 different microorganism by G. pentaphylla extracts.
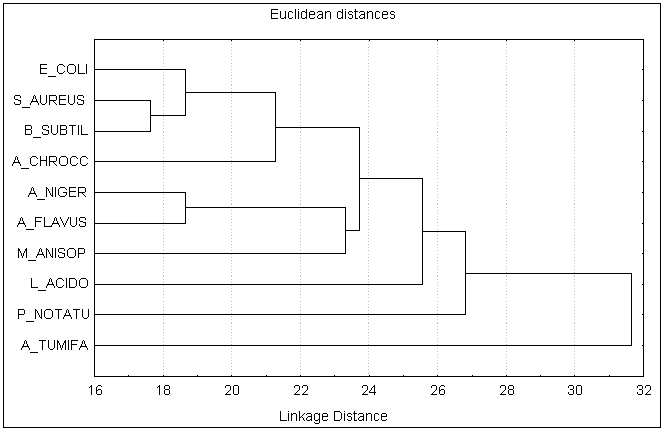
Figure 4. Activity index of G. pentaphylla extracts against bacteria.
LE Ether extract of leaves; LB - Benzene extract of leaves; RE - Ether extract of roots; RB Benzene extract of roots; SE - Ether extract of stems; SB Benzene extract of stems; EE - Ether extract of seeds; EB - Benzene extract of seeds; ; PE - Ether extract of seed pods; PB - Benzene extract of seed pods.
Figure 5. Activity index of G. pentaphylla extracts against fungi.
LE Ether extract of leaves; LB - Benzene extract of leaves; RE - Ether extract of roots; RB Benzene extract of roots; SE - Ether extract of stems; SB Benzene extract of stems; EE - Ether extract of seeds; EB - Benzene extract of seeds; ; PE - Ether extract of seed pods; PB - Benzene extract of seed pods.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||