|
Ethnobotanical Leaflets 14: 559-69. 2010. Antioxidative Potentials of Banana and Plantain Peel Extracts on Crude Palm Oil
1*Arawande Jacob Olalekan and 2Komolafe Eniayo Ayodeji
1Department of Science Lab. Technology, Rufus Giwa Polytechnic PMB 1019, Owo, Nigeria E-mail: 2Department of Food Science and Technology, Rufus Giwa Polytechnic PMB 1019, Owo, Nigeria E-mail:
Issued May 1, 2010
Abstract
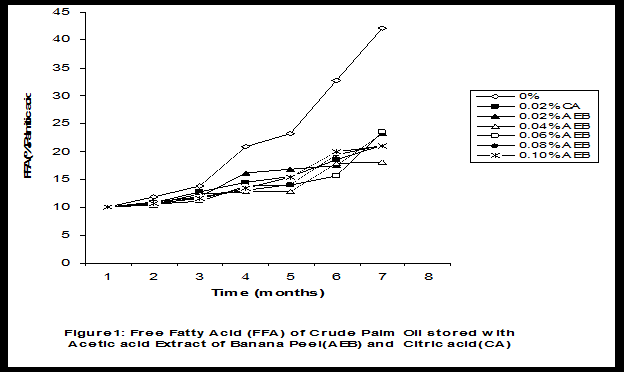
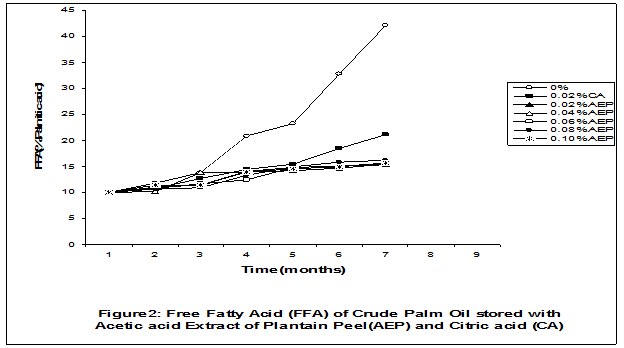
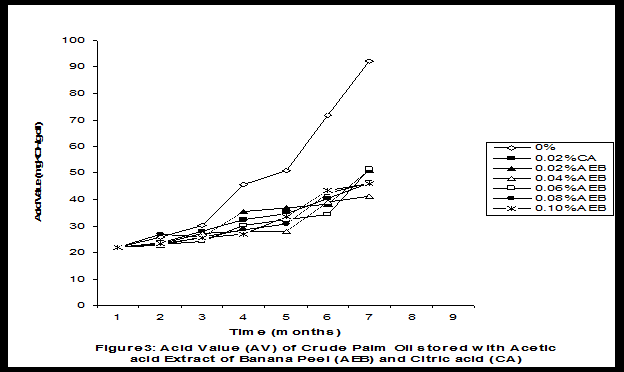
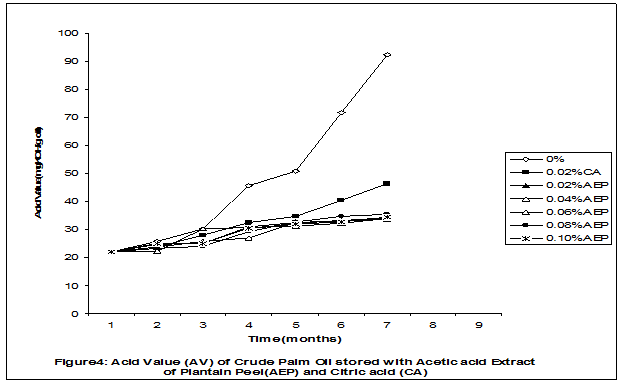
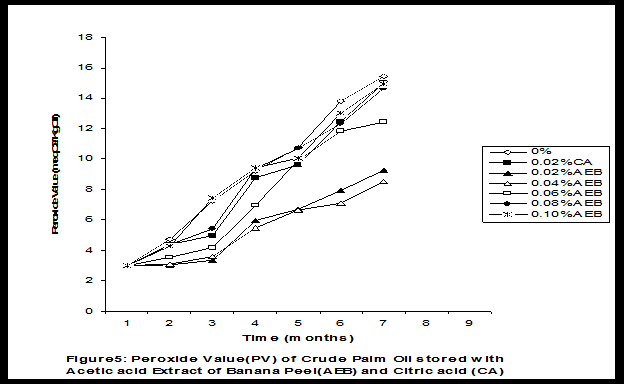
Crude palm oil was obtained from a local palm oil producer at Iyere, Owo, Ondo-State, Nigeria. Ripe peels of banana and plantain were removed from their fruits, cut, sun dried, ground, sieved and separately extracted with five different types of solvents (methanol, acetic acid, ethylacetate, acetone and chloroform). The percent yield of each extract was calculated. The solvent extraction result showed that acetic acid had the highest yield of extract of banana (20.27 0.28%) and plantain (15.840.31%) peels. The lowest yield of extract was obtained with chloroform for banana peel (4.690.13%) and plantain peel (3.390.09%). The acetic acid extracts of banana and plantain peels were separately added at varying concentrations (0.02-0.10%) to crude palm oil stored in transparent plastic containers. Another set of crude palm oil which contained no additive (0%) and 0.02% citric acid was equally set up for comparison. The Free Fatty Acid (FFA), Acid Value (AV) and Peroxide Value (PV) of the oil samples were monitored monthly using standard methods for a period of six months. The results revealed that acetic acid extract of plantain peel had higher antioxidant activity than banana peel against hydrolytic and oxidative rancidity of crude palm oil. The antioxidant activity of both extracts was highest at 0.04% concentration. The extracts showed higher antioxidant activity than 0.02% citric acid in crude palm oil. Key words: Crude palm oil, Banana and Plantain peel extracts, Solvents, Yield, Antioxidant activity. Introduction Crude palm oil is edible oil obtained from African oil palm (Elaeis guineensis). It has been long recognized in West African countries, and among West African peoples it has long been in widespread use as cooking oil (Wikipedia, 2007). European merchants trading with West Africa occasionally purchased palm oil for use in Europe but as the oil was bulky and cheap, palm oil remained rare outside West Africa (Helen, 2007). Before 1880, palm oil constituted the primary export of some West African countries such as Nigeria and Ghana (Wikipedia, 2007). Palm oil was previously the second most widely produced edible oil after soybean oil, 28million metric tons were produced worldwide in 2004 (MOPS, 2005). The oil contains high amount of beta-carotene which makes the oil reddish in colour however the reddish colour turns white when the oil is boiled for few minutes thereby destroying the carotenoids. It is one of the oils relatively high in saturated fats and thus it is a semi solid at room temperature because it contains almost equal proportion of saturated and unsaturated fatty acids content. Palm oil as an edible and cooking oil has been discovered to have an excellent dietary energy source, very rich in vitamins A and E, stable in high temperature (good for frying) and cheap vegetable oil due to the oil palms high productivity (Koh, 2006).However, owing to its high content in saturated fatty acids such as lauric acid, mystritic acid and palmitic acid which are primary cholesterol-elevating fatty acids, palm oil promotes the risk of coronary heart diseases such as hypertension, stroke, heart attach and other cardiovascular diseases (Helen, 2007). In Nigeria and some other African countries where palm oil is commonly found, the oil merchants purchase it when it is relatively cheaper (that is during its season) and store (hoard) for a period of six months or more and later sold it when its price has gone up without considering the deterioration that might have probably set in thereby posing more health risk to the consumers of such oil (Arawande, 2008). Hence there arises the need to prevent oil deterioration by adding antioxidant that will impede the oil rancidity. The use of synthetic antioxidants such as Butylatedhydroxylanisole (BHT), Butylatedhydroxyl toluene (BHT), Propylgallate (PG) and Citric acid to prevent lipid oxidation have been established (Cuvelier et al., 1992; Ruger et al., 2002; Ullah et al., 2003; Khanahmadi and Janfeshan, 2006). But it has been discovered that some of these antioxidants especially BHT and BHA are carcinogenic thereby they are being discouraged in international market as food additives (Carrasquero et al., 1998). This leads to provoking interest in seeking for safer means of natural antioxidants of plant origin that will serve the same purpose of preventing oil rancidity (Tian and White 1994, Erol et al., 2004, Emmanuel and Mudiakeoghene 2008). Banana and Plantain peels are major agricultural wastes which have been used as medicine, animal feeds, blacking of leathers, soap making, fillers in rubber (Hephburn and Blow, 1971; Wikipedia, 2007). Owing to their colours (pigment) when ripe, this suggests that their peels may probably contains antioxidants which can be extracted with suitable solvents since the plants which contain antioxidants are usually coloured. Therefore the focus of this research is to obtain extracts from banana and plantain peels using different solvents, to investigate the antioxidative effects of highest solvent yield extract at varying concentrations on crude palm oil and to compare their antioxidant activities with that of citric acid by monthly monitoring their Free Fatty Acid (FFA), Acid Value (AV) and Peroxide Value (PV) for a period of six months. Materials and Methods The crude palm oil used for this work was purchased from a local palm oil processor in Iyere, Owo, Ondo-State, Nigeria while the banana and plantain fruits were bought from Kings market in Owo, Ondo- State, Nigeria. Preparation and Extraction of Banana and Plantain Peels The ripen peels were removed by hand and cut into smaller pieces for easy drying. The dried peels were ground using electric blending machine and it was sieved with 40mm mesh size. The powdery samples were packed into a black polyethene bags labeled appropriately prior to extraction. Twenty gram of each dried powdery samples were weighed into ten cleaned and dried reagent bottles; and 200ml of each solvent (methanol, ethhylacetate, acetone, acetic acid and chloroform) was separately added to each bottle and left for 72hours during which it was intermittedly shaken on a shaking orbit machine. The mixture was filtered through a 0.45m Nylon membrane filter. The extracts were evaporated to dryness under reduced pressure at 400C by a rotary evaporator. Weight of extract obtained was used to calculate the percent yield of extract in each solvent (Arawande and Abitogun, 2009). Addition of Additives to Crude Palm Oil Acetic acid extract of each peel at varying concentrations (0.02-0.10%) was added to crude palm oil sample contained in transparent plastic containers and it was thoroughly shaken for proper mixing. Crude palm oil containing 0.02% citric acid and that which contain no additive (0%) was also set-up. Each container was labeled appropriately. Chemical Analysis The Free Fatty Acid (FFA), Acid Value (AV) and Peroxide Value (PV) of each oil sample were determined monthly using standard method of analysis (AOCS, 1989) for a period of six months. Results and Discussion The percent yield of banana and plantain peel extract using different solvents is depicted in Table 1. The amount of extract obtained increases as the polarity of the solvent increases. Acetic acid yielded the highest extract in both peels and the yield of methanol extracts was next to acetic acid. The percent yield of extract in banana peel was higher than that of plantain peel in all the solvents used .According to the rule of Thumb, natural antioxidants are polar (phenolic) compounds and they are best extracted using polar solvents (Amir et al., 2005). Ethylacetate, acetone and chloroform yielded about 20-25% extract of the yield of acetic acid in banana and plantain peels. The Free Fatty Acid (FFA) of crude palm oil stored with varying concentration of 0.02%-0.10% acetic acid extract of banana and plantain peels was depicted in Figures1 and 2. It was observed that the plots had similar trend with the plot of free fatty acid of pistachio nut stored under various atmospheric and temperature conditions as reported by Maskan and Karatas (1998). The FFA of crude palm oil stored with varying concentration of banana and plantain peel extracts were lower than the values obtained for oil sample that contained no additive (%) in all the six month period of storage. It was obviously noticed that the FFA of crude palm oil stored with 0.02%-0.10% plantain peel extract was lower than that of the oil sample stored with 0.02% citric acid and 0.02%-0.10% banana peel extract especially at the last three months of storage. Figures 3 and 4 respectively showed the Acid Value (AV) of crude palm oil stored with varying concentration of 0.02%-0.10% of acetic acid extract of banana and plantain peels. The trend observed above for FFA was also the same with that of Acid Value in all the storage conditions only that the AV values were higher than that of FFA. Ihekoronye and Ngoddy (1985) reported that the FFA and AV of any lipid were both measure of hydrolytic rancidity and that the lower their values, the slower was the rate of hydrolytic rancidity. Hence crude palm oil stored with varying concentration of 0.02%-0.10% acetic acid extract of banana and plantain peels were less prone to hydrolytic rancidity even more than 0.02% citric acid. This showed that the extracts at varying concentration demonstrated high antioxidant activity. However the antioxidant activity of plantain peel extract was higher than that of banana peel extract against hydrolytic rancidity of crude palm oil. The Peroxide Value (PV) of crude palm oil stored with varying concentration of acetic acid extracts of banana and plantain peels are shown in Figures 5 and 6 respectively. The plots obtained had similar trend with the plot of peroxide value of pistachio nut and influence of garlic extract on the oxidation process of edible oil (Maskan and Karatas 1998, Zalejiska-Fiolka 2001). It has been reported that peroxide value is a measure of oxidative rancidity or stability of lipid and that high peroxide value of lipid is an indication of high occurrence of oxidative rancidity (Gunstone and Norris 1983, Rossel 1994, Maskan and Karatas 1998, Amir et al., 2005). In all the months of storage, crude palm oil stored with 0.02%-0.10% plantain peel extract had lower values of PV than oil sample stored with 0.02% citric acid. 0.02%-0.06% banana peel extract also lowered the PV of oil sample than 0.02% citric acid in all the storage months. Plantain peel extracts showed higher antioxidant activity in crude palm oil sample than banana peel extracts, although both extracts demonstrated higher antioxidant potential against oxidative rancidity of crude palm oil sample than 0.02% citric acid expect at 0.08% and 0.10% banana peel extract. Considering the effect of concentration of extracts, the antioxidant activity of plantain peel extract against oxidative rancidity of crude palm oil decreases in the order of 0.04%0.06%0.02%0.08%0.10% while the antioxidant activity of banana peel extract against oxidative rancidity of crude palm oil decreases in the order of 0.04%0.02%0.06%0.0%.%. The optimal antioxidant activity of both peel extracts was 0.04% while 0.08% and 0.10% of banana and plantain peel extract gave the least antioxidant activity in crude palm oil. In conclusion, plantain and banana peel extract was highest in acetic acid and the acetic acid extracts of these peels had pronounce antioxidant activity against both hydrolytic and oxidative rancidity of crude palm oil stored in transparent plastic containers. Their antioxidant activity was much higher at 0.04% than 0.02% citric acid. However further research can be conducted using refined vegetable oils stored in tin and glass containers. The antioxidant activity of water and methanol extracts of banana and plantain peels can also be investigated at varying concentrations on crude and refined edible oils over a period of twelve months. References Amir, H.G., Moshshen, B. and Mohammed, A.S. (2005). Antioxidant activity and total phenolic compounds of pistachio hull extracts. Food Chemistry, 92: 531-535. AOCS (1989). Official and Tentative Method of American Oil Chemists Society, 4th Ed. American Oil Chemists Society, Champaign, U.S.A; Method 8, 53. Arawande, J. O. (2008). Effect of storage containers on shelf life of refined soybean oil, Int. J. Pure & Applied Sci 1:76-81. Arawande, J.O. and Abitogun, A.S. (2009). Comparative studies of antioxidant potential of citric acid and methanolic extract of cabbage star leaf on crude palm kernel oil, J .Chem. Soc. Nigeria 34(1):54-57. Carrasquero, A., Salazar, M. and Nava, P. B. (1998). Antioxidant activity of grape seed extract on vegetable oils, J. Sci. Food & Agric., 77:436-467. Cuvelier, M. E., Richard, H. and Berset, C. (1992). Comparison of antioxidant activity of some acid phenols: Structure activity relationship. Biosci. Biol. Tech. Biochem., 56:324-326 Emmanuel, O. A. and Mudiakeoghene, O. (2008). The use of antioxidants in vegetable oil-A review. African J. Biotechnology 7(25): 4836-4842. Enrol, D., Mechmet, U., Ferda, C., Dimitra, D., Gulhan, V.U. Mosschos P. and Atalay, S. (2004). Antimicrobial and antioxidantive activities of essential oils and methanol extract of Saliva cryptantha (Montbret et Aucherex Benth) and Saliva multicaulis (Vahl), J. Food Chemistry, 84: 519-525. Gunstone, F. D. and Norris, F. A. (1983). Lipid in Food: Chemistry, Biochemistry andTechnology, Pergamon Press, New York, 58-63 Ihekoronye, A.I. and Ngoddy, P.O. (1985). Integrated Food Science and Technology for the Tropics, Macmillian Publisher Limited, London, 67-78. Helen, B. (2007). The oil for Ape scandal: How palm oil is threating the orangutan.Friends of the Earth. Retrieved on 29/09/2007. Hephburn, C. and Blow, C. M. (1971). Rubber Technology and Manufacturing, 3rd Ed. Butterworth Publication 188. Khanahmadi, M. and Janfeshan, K. (2006). Study on antioxidation propertyof Ferulago angulata Plant. Asian J. Plant Sci 2(17-24): 521-526. Koh, C. S. (2006). Commets on draft document: Diet, nutrition and other prevention of chronic diseases. http://www.who.int/dietphysicalactivitymedia/en/gsfao_cmo_068.pdf. Maskan, M. and Karatas, S. (1998).Fatty acid oxidation of Pistachio nuts stored under various conditions and different temperature, J. Sci. Food Agric., 77:334-340. MOPS (2005). Malaysian Oil Palm Statistics, Malaysian Palm Oil Board, Malaysia. Rossel, J.B.1994. Measurement of Rancidity. In: J.C.Allen and R.J.Hamiton (Eds.), Rancidity in Food, 3rd Ed. Balckie, U.K. Ruger, C.W., Klinker, E.J. and Hammond, E.G. (2002). Abilities of some antioxidants to stabilize soybean oil in industrial use conditions. J.Am. Oil Chem. Soc. 79(7): 733-736. Tian, L. L. and White, P.J. (1994). Antioxidant activity of Oat extracts in soybean and cottonseed oils. J. Am. Oil Chem. Soc 71: 1079-1086. Ullah, J., Hamayoun, M., Ahmed T., Ayub, M. and Zarafullah, M. (2003).Effect of light,natural and synthetic antioxidants on edible oils and fats. Asian J. Plant Sci. 2(17-24): 1192-1194. Wikipedia 2007. Palm Oil. http://en.wikipedia.org/wiki/palmoil. Retrieved 24/07/2007. Zalejiska-Fiolka, J. (2001).The Influence of Garlic extract on the oxidation process of edible oils, La Rivista Italian Delle Sostanza Grass, 78:343.
Table1: Percent Yield of Banana and Plantain Peel Extract using different Solvents.
Mean of triplicate determinations Standard deviation
|
||||||||||||||||||||||||||||||||||||||||